Objective: To quantify and provide objective data determining tissue volume changes over time after a stem cell facelift, and to measure its effectiveness in volume augmentation.
Methods: A prospective multi-case study of patients who underwent a stem cell facelift was analysed using three-dimensional (3D) images of preoperative and postoperative time-points to assess changes in facial volumes over time. A Vectra H1 (Canfield Imaging Systems, Fairfield, NJ) camera and software suite were used to measure the change in volume.
Results: The study included seven patients (14 hemi-faces) comprising two females and five males, with mean age of 64.72 ± 17.58 years. Overall, there was an 83% volume improvement across all patients. Wilcoxon signed ranks tests was used to determine whether there was a significant change in the distances (mm) before and after the procedure. The percentage increase was proven statistically significant (95% confidence, Z=-3.2958, P=0.00096<0.05), indicating an effectiveness of the stem cell treatment on the facelift among the seven patients.
Conclusions: This study tracked the changes of volume seen after a stem cell facelift and provided significant evidence of tissue regeneration, even as a small case study. Overall, there was an 83% volume improvement seen in patients.
Stem cells are undifferentiated cells that have the capacity to self-renew and give rise to specialised types1. Autologous sources of stem cells are the safest form of stem cells2 owing to the absence of graft versus host response, as the donor and recipient are the same person. A study in 20093 showed that when stem cells were combined with hyaluronic acid they were able to fill-in deep folds, with progressive improvement of skin tone and decreasing lines of expression. Another study in 2009 by Kim4 showed that both increasing dermal thickness and collagen density after adipose-derived stem cell (ADSC) injections into photodamaged and aged skin led to a reduction of wrinkles. A more recent study in 2013 by Piccinno et al5, demonstrated the ability of adipose stromal cells (ASC) to help protect transferred adipose tissue from necrosis, while concomitantly increasing vasculogenesis.
Within the last few years, there has been an explosion of interest and research geared towards new stem cell therapies. While there are a number of studies showing stem cell effectiveness in animals, few have been performed in humans. As a relatively new science, there are seemingly no current standards for treatment.
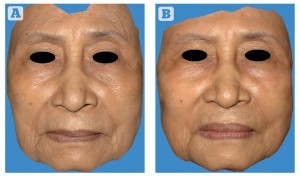
Figure 1 Patient 2 — transformed three-dimensional image between (A) preoperative baseline image and (B) 7-months after the stem cell facelift procedure
The effectiveness of using stem cells for aesthetic improvement is currently lacking real clinical objective data in the literature. According to a joint position statement regarding stem cells and fat grafting released by both the American Society for Plastic Surgeons (ASPS) and the American Society for Aesthetic Plastic Surgery (ASAPS), there is a need for more evidence-based medicine6. This article and the study it summarises will serve as a preliminary guide and first-line evidence for succeeding studies regarding the use of cellular procedures for facial rejuvenation.
The objective of the study was to quantify and provide unbiased data demonstrating tissue volume changes over time after a stem cell facelift using a VectraH1 (Canfield Imaging Systems, Fairfield, NJ) three‑dimensional (3D) camera and software suite. Furthermore, the study was performed with a standardised protocol for stem cell harvesting, isolation, transplant, and tracking of volumetric changes over time in order to know whether tissue regeneration had transpired. This protocol is currently the best way to physically demonstrate the success or failure of the stem cell facelift in an aesthetic clinic.
Patients and methods
Patients who were already undergoing an autologous cellular procedure were selected to participate in the study. Prior to providing the autologous treatment, informed consent was procured from all patients following an in-house patient edification about stem cells and expectations, the clinical and laboratory aspects of a stem cell facelift, and an ethical review. Additionally, all patients underwent a thorough screening and evaluation. A complete history and physical examination were given. Laboratory tests, current blood chemistry, medications, imaging reports, and clearance prior to the procedure were all completed. Patients undertook the procedure willingly and the treatment was provided free of charge at the author’s private clinic.
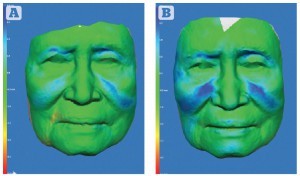
Figure 2 Patient 2 — three-dimensional colourimetric analysis demonstrating areas of volume change (blue) over time. Increasing depth of blue represents increased volume. Between (A) 2 months and (B) 7 months post‑procedure. The 83 year-old female was very pleased with the results
A standard manual harvesting technique using tumescent anaesthesia7, with a Toomey syringe8 (Bard Medical Division, Covington, GA) was performed. The donor fat came from the patients’ own abdomens and flanks. The collection and harvesting of the stromal vascular fraction from adipose was performed as known to those in the art9. The cellular product was concentrated by centrifugation and provided in a concentration of 1.5 ml of cellular stromal vascular fraction (SVF), containing approximately 100 million cells, combined with 3.5 ml of growth factors. This was then divided in two and used to inoculate the autologous unpacked adipose into a complete cellular mixture. The term ‘ADSC matrix’ was used to connote this mixture.
The preparation of stromal vascular cells from the lipoaspirate was obtained using standard protocols10, either from the abdomen, flanks, or thighs. The lipoaspirate was first washed thoroughly in sterile physiological saline or sterile phosphate buffered saline (PBS) before being subjected to enzymatic digestion using sterile collagenase, deactivating the enzymatic reaction with an autologous serum, and further separation in order to obtain a single-cell suspension. After digestion, the centrifuged cell pellet, termed the SVF, was suspended in an expansion medium before serial filtration through micrometre nylon filters. The content of the cells was then counted using a Scepter™ Cell Counter11 (EMD Millipore, Billerica, MA), and qualitatively analysed via a haemocytometer.



