Dr Firas Al-Niaimi explains why he trusts in the latest vascular laser platform from Candela
The first working laser was developed in 1960 by T.H. Maiman, using a rod of ruby to emit photons of light1. In that same decade, many other lasers were being invented and trialled in medicine and, in 1963, dermatologist Leon Goldman used the first lasers in dermatologic practice. These included the ruby, argon, and carbon dioxide continuous-wave lasers2. In 1981, a collaboration with John Parish — then the chairman of dermatology at Harvard University — resulted in the first working vascular dermatology laser (by Candela); a flash lamp pumped organic dye laser3. The medium used within a flash pump laser was a fluorescent dye that was housed in a transparent cell and powered by a flash lamp, emitting a wavelength of 577 nm3.
Further research led to the discovery of a new wavelength — the 585 nm instead of the 577 nm — only to be replaced later by 595 nm, which is still used in the Candela Vbeam laser and in the newest model Vbeam prima™4. Studies conducted by Candela showed that by shifting the wavelength from 577 nm to 585 nm, and eventually to 595 nm, resulted in a preferential depth of penetration, together with increased safety on epidermal melanin; albeit slightly at the cost of higher fluences requirement5. Several dyes were considered and used, and these included fluorescein, coumarin, stilbene, tetracene, and umbelliferone6. Currently, the Vbeam family PDLs use rhodamine dye due to its efficiency and relative long life-time.
Relevant laser physics
The term ‘laser’ is an acronym for ‘Light Amplification of Stimulated Emission of Radiation’, and is based on the concept of electron stimulation which results in the release of photonic energy7. The ultimate light and tissue interaction that takes place results, one way or another, in a biological response. This response can broadly be subdivided into thermal, chemical, and mechanical (acoustic). Putting it in very simplistic terms; the thermal effects rely on the heat that is produced by the laser energy to either selectively or non-selectively target a tissue component. An example is the coagulation of a vessel.
Photochemical effects occur due to the up- or down-regulation of specific biological pathways, occurring as a consequence of light and tissue interaction8. This photochemical effect may not necessarily be related to the generation of ‘heat’ and may require the addition of a photosensitiser (a topical drug), a phenomenon called photodynamic therapy. An example of a photochemical response is the tissue effects on inflammatory dermatological conditions following the use of PDL. Lastly, photomechanical effects occur predominantly due to the acoustic effects on the tissue (though thermal effects due to the laser energy play a role). This is caused by the combination of short rapid pulses and a rapid peak in the energy that is produced in tissue as a result leading to a response called ‘cavitation’. An example of this is the rupturing of blood vessels giving rise to ‘purpura’ when using a very short (example 0.45 millisecond) pulse duration with the PDL in the treatment of vascular malformations9.
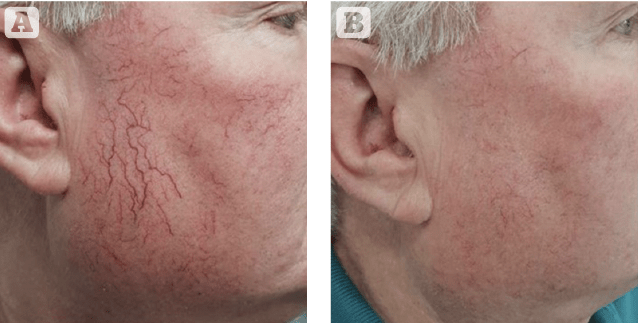
Figure 1 Photos taken (A) before and (B) after treatment with the 3 x 10 mm spot on individual vessels followed by the novel 15 mm spot and a single pass on 9 mm. This is the ‘layered approach mentioned in the text. Results are from a single treatment
The Vbeam prima™ is a vascular laser that has dual wavelengths 595/1064 nm and therefore primarily targets haemoglobin, which is present in the red blood cells circulating in blood vessels, and acts predominantly through photothermal effects leading to vessel coagulation. Haemoglobin shows absorption peaks in the blue, green, and yellow bands (414, 542, and 577 nm), as well as a peak in the near-infrared portion of the spectrum (700 to 1100 nm)10. It is important to note here that these peaks relate mainly to the oxygenated form of haemoglobin — the so-called oxyhaemoglobin10. Other forms of haemoglobin, such as deoxyhaemoglobin and methaemoglobin, have other absorption spectra, such as in the near-infrared range hence the 1064 nm Nd:YAG laser in the Vbeam prima™ can successfully target venous blood that is rich in deoxyhemoglobin. Depth of penetration of light originating from a laser is proportionate to its wavelength (up to 1200 nm) and therefore given the longer wavelength of 1064 nm it can reach deeper vessels, such as leg vessels and venous malformations11. The absorption of 1064 nm Nd:YAG is almost a tenth that of 595 nm, hence higher fluences are required for an optimal biological response.
In clinical practice, this means that the incoming pulsed laser will be absorbed by the circulating haemoglobin in the vessels that transform this energy to heat which radiates to the vascular wall and results in heating the endothelial vessel wall. In essence, it is the endothelial wall that is the ultimate ‘target’ of the vascular laser, in treating vessels. As described briefly above, the photothermal effects of the laser can either be selective or non-selective. In the selective case, confinement of the response to the chosen target (in this case, the vessel) should occur without any collateral damage to the surrounding tissue. In order to achieve this, the pulse duration here plays an important role, and this is based on the so-called ‘selective photothermolysis’ theory founded by Anderson and Parish, which revolutionised the modern use of lasers in dermatology12. An example of a non-selective photothermal effect of lasers is the use of carbon dioxide lasers, which would ablate tissue and cause non-selective heating.
Features of the Vbeam prima™
The Vbeam prima™ is a comprehensive and advanced vascular platform which currently represents the most sophisticated vascular platform in medicine. The advanced and enhanced features compared to the previous model — the Vbeam perfecta™ — relate to wavelength, spot size, cooling methods, software, and dye properties. Each feature will be explained in more detail below.
Wavelengths
The Vbeam prima™ has the two most efficient wavelengths for vascular treatments; the 595 and 1064 nm. The addition of the 1064 nm Nd:YAG has come with significant benefits; namely the enhanced efficacy of treating all vascular conditions with the advantage of the depth and preferential response to the Nd:YAG wavelength for deep and venous lesions such as venous lakes, venous malformations, and venules. It is important to appreciate the importance of pulse duration with the Nd:YAG. The ms pulse duration is particularly important for vascular lesions with its width related to the vessel diameter or amount of chromophore, typically long pulse durations for larger vessels. The Vbeam prima™ also has the option of a microsecond (μs) pulse duration in its Nd:YAG handpiece, also referred to as ‘sub-millisecond’). This is particularly exciting as it primarily targets dermal water leading to increased dermal heating with various benefits from rejuvenation and tissue tightening to pore size reduction and even inflammatory acne and diffuse redness13,14. An example of this technology exists by another manufacturer as ‘laser genesis’, and I would like to coin the term ‘sub-millisecond Nd:YAG remodelling’ for this feature in Vbeam prima™.
Spot size
The spot size is an important variable in lasers as it determines the depth of penetration as well as coverage. Three unique spot size features exist with the Vbeam prima™. The first is the unique to Candela 3 x 10 mm elliptical spot size, which specifically targets linear vessels with little to no effect on the surrounding tissue, therefore optimising results and specifying treatments to unwanted telangiectasia or venules15. This unique elliptical spot functions in both the 595/1064 nm wavelengths.
The second feature is the new large spot size of 15 mm, which in addition to the speed of coverage (think of a large port-wine stain on the body) it is particularly advantageous when depth is required as larger spots penetrate deeper and therefore have an enhanced biological activity with the deeper targets. One of the limitations we faced in the treatment of certain vascular conditions such as port-wine stains is the limitation in the depth of laser light obtained from existing devices which posed the difficulty for optimal treatment (the Nd:YAG may not be suitable in certain conditions given the high adverse risks associated for particular treatments). Having the largest vascular laser spot size in medicine (15 mm) represents a new exciting era of targeting deep vessels — previously not possible with the PDL — leading to optimisation in clinical response. With a larger spot size, there is less scattering of light and more efficient photonic energy delivered at a deeper level. Evidence of the efficiency of the 15 mm unique spot size has already been published by Bernstein et al. in both rosacea and poikiloderma16,17. The author has already experienced a marked response in the treatment of port-wine stains, thick scars, and rosacea utilising the 15 mm spot followed by a second pass (or in subsequent treatments) with a smaller spot to gradually reach more superficially located vessels; a technique the author refers to as ‘layered approach in vascular treatments’.
The third and last advantage of the current spot size is the ability to adjust the spot diameter by increments of 0.5 mm, allowing for exact customisation of targeted treatments without unnecessary targeting of the surrounding skin.
Cooling methods
Cooling is an important parameter in protecting the epidermis as well as reducing pain and discomfort. Adequate epidermal cooling reduces the risk of crusting, blistering and/or dyspigmentation. Adjustment of cooling is required with variables of the fluence used and concentration of melanin present.
Cooling of the epidermis can either be achieved by cold air, contact, or cryogen spray cooling. A sophisticated technology enabling a spatial cooling confined predominantly to the epidermis is the dynamic cooling device (DCD), which allows for a cooling spray of tetrafluoroethane followed by a delay period before the laser is fired on the skin. This allows for spatial cooling of the epidermis, without significant vessel cooling, that may lead to vasospasm with reduced circulating chromophore as a consequence18. The DCD technology is only found in Candela devices.
The Vbeam prima™ has an additional method of cooling — contact cooling — referred to as ‘EverCool™’. This unique handpiece has a transparent window in which the spot size can be adjusted within and is chilled through a continuous flow of cooled water flowing through adjacent metal at the edges of the window. The temperature can be adjusted according to the variables of fluence, chromophore, and melanin content to ‘cold = 20° Celsius’, ‘colder = 15° Celsius’, and ‘coldest = 10° Celsius’. In the author’s experience with the device, the EverCool™ handpiece is particularly desirable for dyschromia, sensitive skin, and with higher fluencies using the Nd:YAG wavelength. A serendipitous use of the handpiece by the author is when compression of a target is required to blanch or reduce the chromophore density such as with venous lakes, large venous malformations, and port-wine stain blebs.
Dye and interface features
The rhodamine dye in the upgraded device is much more efficient and longer-lasting than the already existent model in the Vbeam perfecta™. This efficiency and longevity stems from an enhanced way of stimulating the light through less rhodamine use and additional flash-lamps used for excitation. The software has a meter to track the amount of dye left, which is particularly useful in anticipating the need for replacement.
The interface is comprehensive with a drop-down menu to aid the doctor in choosing the right parameter based on the treated pathology features. The system can be remotely accessed through Wi-Fi connectivity, which is an added advantage to enhanced customer service.
Optimising vascular treatment
The ultimate goal in vascular laser treatment is to induce vessel wall damage leading to vessel clearance with minimal collateral damage to the epidermis or surrounding tissue. Although haemoglobin is the chromophore or absorbing target of the PDL, radial diffusion of the generated heat will ultimately lead to the vessel wall lining being targeted. Scientific studies have demonstrated that the vascular lining (endothelium) needs to be sufficiently heated to a temperature of around 65–70° Celsius for at least one ms in order to cause denature of structural proteins leading to vessel closure and clearance19. Therefore, in this case, the fluence chosen and the pulse width are very important parameters. Too much heat delivered can result in collateral damage or a rapid rise in intravascular temperature that leads to steam formation and vessel rupture, clinically evident as purpura. Very short pulse durations lead additionally to a photomechanical effect again leading to vessel rupture and purpura20. In summary, vessel clearance can be achieved through a photothermal (choice of fluence and pulse width should get the endothelium temperature to around 65–70° Celsius for at least one ms) or photomechanical effect (short pulse width with sufficient energy leading to vessel rupture). Vessels have different diameters, and therefore, the ‘length’ that is required for the heat to diffuse and reach the vessel wall is variable and is termed the ‘thermal diffusion length’15. It is therefore apparent that larger diameter vessels need more time for the diffused heat to reach the vessel wall compared to a smaller diameter vessel. Clinically, this is reflected in the chosen pulse duration.
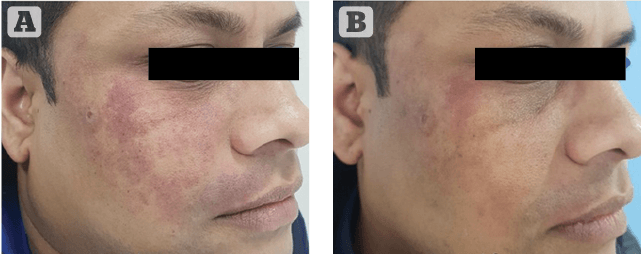
Figure 1 Photos taken (A) before and (B) after single treatment marked results in port-wine stain using the EverCool contact cooling handpiece with 15 mm spot at purpuric setting
In practice, this means that if the chosen fluence is too low, insufficient heating of the endothelium occurs and vessel closure will not take place. In my experience, most of the individuals seeking treatment for vessels and diffuse erythema do not desire to have purpura and, therefore, one should optimise the photothermal effect of vessel coagulation rather than vessel rupture in this case. Pulse stacking or multiple passes often add a beneficial effect due to the stepwise rise in temperature formation leading to vessel clearance.
Leg telangiectasia greater than 0.3 mm generally tend to lie deeper than the reach of PDL and are preferably treated with the 1064 nm Nd:YAG. This is because of its deeper penetration profile and relative selectivity for deoxyhaemoglobin. In general, blue and purple vessels contain more blood (larger diameter) and therefore a high chromophore content that requires fewer fluences compared to small red vessels that contain less chromophore and, therefore, need higher fluences for coagulation.
Furthermore, leg veins are generally harder to treat compared to facial telangiectasia due to their increased hydrostatic pressure, thicker vessel wall, and the fact they contain less oxyhaemoglobin as a chromophore (particularly the blue and purple vessels).
Understanding and recognising the clinical endpoints in vessel treatment is essential for optimal results. These include a colour change in the vessel (often darkening), vessel blurring, low refill rate (gentle compression on the vessel does not lead to a refill, which indicates coagulation of the vessel), and vessel disappearance. Erythema with subsequent oedema is a normal reaction afterwards. Greying or whitening are ominous signs and imply an epidermal injury with the risk of crusting and/or blistering, and the fluences should be lowered subsequently21. It should also be noted that purpura on the limbs tend to clear more slowly compared to the face and often results in post-inflammatory hyperpigmentation. Unless indicated per condition, it is, therefore, preferable to treat vessels off the face in a non-purpuric mode.
Non-primary vascular conditions treated by the Vbeam prima™

Conclusion
The Vbeam prima™ currently represents the most advanced vascular laser platform with dual wavelengths 595/1064 nm and enhanced features to deliver an unmatched service in vascular lasers. The presence of a large spot size (15 mm) with dual methods of cooling and a tuneable pulse width allows for enhanced efficacy in the treatment of deeper vessels as well as higher skin types and dyschromias.
Declaration of interest Funding for submission of this paper was provided by Candela
Intro image © Shutterstock.com, Figures 1–2 © Dr Firas
- Alster TS. Manual of cutaneous laser techniques. Lippincott Williams and Wilkins; 2000. Preface; p. 11.
- Tanzi EL, Lupton JR, Alster TS. Lasers in dermatology: four decades of progress. J Am Acad Dermatol 2003;49:1-31.
- Source: International Directory of Company Histories, Vol. 48. St. James Press, 2003.
- Wall TL. Current concepts: Laser treatment of adult vascular lesions. Semin Plast Surg 2007;21:147-158.
- Bernstein EF, Lee J, Lower J, et al. Treatment of spider veins with the 595 nm pulsed-dye laser. J Am Acad Dermatol 1998;39(5 Pt 1):746-750.
- Libertini LJ, Small EW. On the choice of laser dyes for use in exciting tyrosine fluorescence decays. Anal Biochem. 1987;163(2):500-5.
- Sakamoto FH, Wall T, Avram MM, Anderson RR. Lasers and flash lamps in dermatology. In: Wolff K, Goldsmith LA, Katz SI, et al (eds). Fitzpatrick’s dermatology in general medicine (7th ed). New York: McGraw-Hill, 2008:2263-78.
- Weber RJ, Taylor RB, Engelman DE. Laser-induced tissue reactions and dermatology. Curr Probl Dermatol. 2011;42:24-34.
- Trelles MA, Svaasand LO, Verkruysse W, et al. Purpura without vessel structural damage. Lasers Med Sci. 1998;13(4):299-303.
- Van Gemert MJ, Henning JP. A model approach to laser coagulation of dermal vascular lesions. Arch Dermatol Res 1981;270(4):429-39.
- Bogdan Allemann I, Kaufman J. Laser principles. Curr Probl Dermatol. 2011;42:7-23.
- Anderson PR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science 1983;220(4596):5247.
- Schmults CD, Phelps R, Goldberg DJ. Nonablative facial remodeling: erythema reduction and histologic evidence of new collagen formation using a 300-microsecond 1064-nm Nd:YAG laser. Arch Dermatol. 2004;140(11):1373-6.
- Koh BK, Lee CK, Chae K. Photorejuvenation with submillisecond neodymium-doped yttrium aluminium garnet (1064 nm) laser: a 24-week follow-up. Dermatol Surg 2010;36(3):355-62.
- Bernstein EF, Kligman A. Rosacea treatment using the new-generation, high-energy, 595 nm, long pulse-duration pulsed-dye laser. Lasers Surg Med. 2008;40(4):233-9.
- Bernstein EF, Schomacker K, Paranjape A, Jones CJ. Pulsed dye laser treatment of rosacea using a novel 15 mm diameter treatment beam. Lasers Surg Med. 2018;50(8):808-812.
- Bernstein EF, Schomacker K, Paranjape A, Jones CJ. Treatment of poikiloderma of Civatte using a redesigned pulsed dye laser with a 15 mm diameter treatment spot. Lasers Surg Med 2019;51(1):54-58.
- Nelson JS, Milner TE, Anvari B, et al. Dynamic epidermal cooling during pulsed laser treatment of port-wine stain. A new methodology with preliminary clinical evaluation. Arch Dermatol. 1995;131(6):695-700.
- Suthamjariya K, Farinelli WA, Koh W, Anderson RR. Mechanisms of microvascular response to laser pulses. J Invest Dermatol. 2004;122(2):518-25.
- Garden JM, Tan OT, Kerschmann R, et al. Effect of dye laser pulse duration on selective cutaneous vascular injury. J Invest Dermatol. 1986;87(5):653-7
- Nanni C. Complications of laser surgery. Dermatol. Clin 1997;15(3):521-34.
- Forbat E, Al-Niaimi F. Nonvascular uses of pulsed dye laser in clinical dermatology. J Cosmet Dermatol. 2019 [Epub ahead of print].
- Erceg A, de Jong EM, van de Kerkhof PC, Seyger MM. The efficacy of pulsed dye laser treatment for inflammatory skin diseases: a systematic review. J Am Acad Dermatol. 2013;69(4):609-615.
- Liu A, Moy RL, Ross EV, et al. Pulsed dye laser and pulsed dye laser-mediated photodynamic therapy in the treatment of dermatologic disorders. Dermatol Surg. 2012;38(3):351-66.
- Omi T, Kawana S, Sato S, et al. Cutaneous immunological activation elicited by a low-fluence pulsed dye laser. Br J Dermatol. 2005;153 Suppl 2:57-62.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018 [Epub ahead of print].
- Piccolo D, Kostaki D, Del Duca E, et al. Long-pulsed 1064-nm Nd:YAG laser for the treatment of onychomycosis. Photomed laser Surg 2017;35(4):213216





