Patrizia A d’Alessio explains how the gut-brain relationship can be the cause and possible solution to issues around inflammation and skin ageing

email [email protected]
What will be covered in this article? Well, we will discuss the relationship between the visible and the invisible. If the dialogue between the gut and the brain is invisible, the progressive transformation of our skin is clearly perceptible and therefore, anxiously scrutinised. In simple terms, we are constantly observing the outcomes of our gut-brain relationship, and we cannot avoid the impression that our skin is losing its allure.
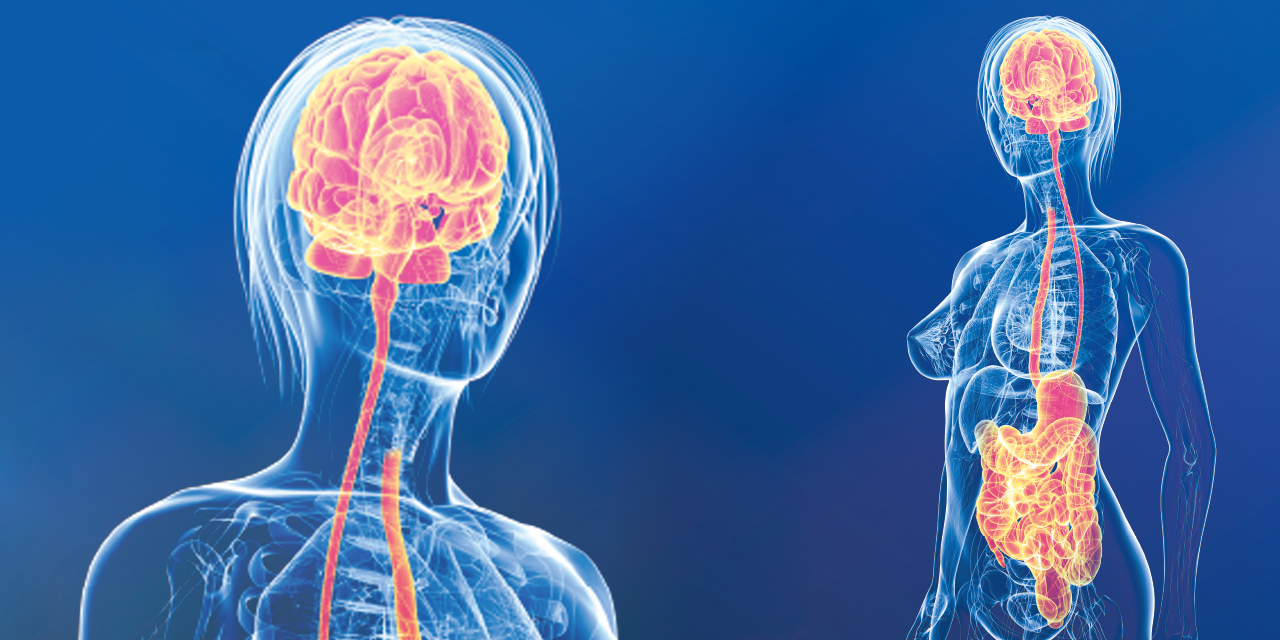
Let us begin with anatomy, not of the brain of course, nor the skin, but of the connections between the two strategic organs that determine outcomes for general health as well as specific functionalities of the skin. Indeed, the anatomical gut-brain link is ensured by the vagus nerve, whereas the functional gut-brain link relies on the gut microbiota and its signalling.
Bidirectional communication channels are, therefore, activated continuously between the gut and the brain. Endocrine-, neurocrine- and inflammation-related signals generated by the gut microbiota and their specialised cells affect the brain. In turn, the brain can influence the microbial composition and functions via endocrine and neural mechanisms. Substances such as dopamine, serotonin, leptin or adiponectin are present in the brain as well as in the gut. However, their release appears better represented in the gut than in the brain. Moreover, serotonin- and dopamine-releasing bacteria have also been described in the gut1. In other words, an individual’s quality of life (QoL) and the feeling of reward/pleasure would seem to be more dependent on the gut’s intrinsic machinery, than the secretion of the brain’s neurotransmitters.
In order to better understand the gut-brain connection, it is necessary to consider:
- The issue of food intake and the consequent signalling by micronutrients
- The role played by the brain and the gut immune system in stress management, as stress profoundly alters the structure of the gut barrier and can compromise its function resulting in dysbiosis
- Reaching the visible side of this complex sequence of events relevant to skin health and beauty.
To summarise, because food intake activates complex signalling pathways, it places itself as the starting point between skin youth and its relationship with the gut.
The gut as a starting point
In ancient times, and notably since the 17th and during the 18th century, dietary components have been observed to have specific effects on general health and skin quality alike. Vegetarianism became a treatment for obesity and gout, plaguing wealthy people consuming meat excessively. Dr George Cheynes (1671–1743), who launched vegetarianism, was also beginning to consider it as a solution to avoid wrinkles and lack of skin radiance2. Today, we know that specific food components of meat or vegetables initiate gut-brain communication. One of the first steps of this dialogue is the recruitment or preferential enhancement of specific microbiota strains devoted to the digestion of these foods that we are incapable of processing ourselves. The production of short-chain fatty acids (SCFA) is thereby an unavoidable strategic step3.
To continuously perform these tasks, the gut landscape itself has been defined as a ‘continuously perfused and peristaltic bioreactor facing a functional anti-inflammatory barrier’4. Moreover, the production, by our enterocytes, of 10 litres of mucus per day and the presence of a thin film of glycocalyx atop enterocytes, both reinforce the physical barrier between the microbiota circulating in the gut lumen and the mucosa, while allowing microbiota secretions to enter the lamina propria.
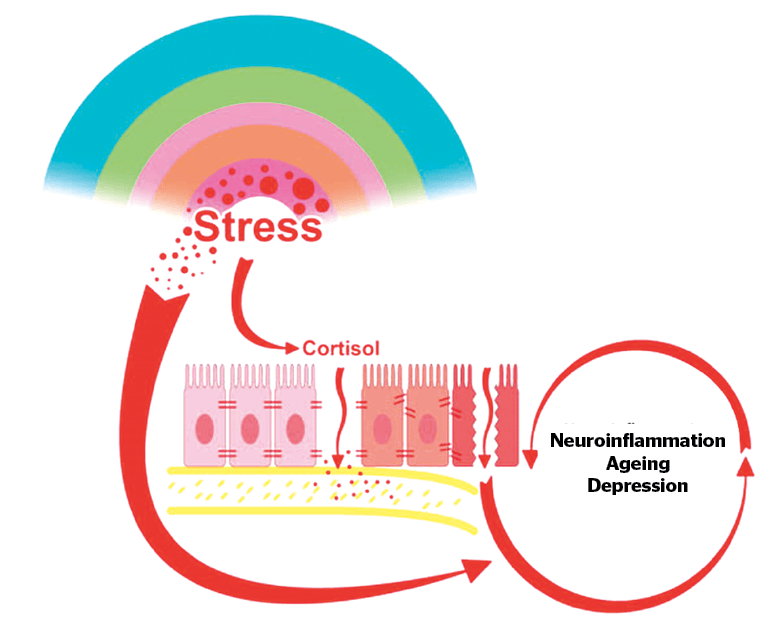
Figure 1 As the brain attempts to cope with ongoing stress, a sustained production of cortisol takes place. This ultimately alters the intestinal barrier, which looses its tightness, resulting in a ‘leaky gut’ also known as ‘dysbiosis’. The consequence is a generalization of chronic inflammation and finally neuro-inflammation, translated in ‘sickness disease’
As the mouth’s microbial micro-environment is continually communicating with its gut counterpart5, during food consumption, specific strains of the microbiota are selected. The metabolites, generated through the digestive process by the bacteria finalised in the production of SCFA, have been shown to be crucial for life-sustaining issues, such as tissue and membrane repair, and anti-inflammatory and immune-stimulating activities. Futhermore, acetate, butyrate, folate and propionate are produced by Bifidobacteria, Clostridium, Enterobacterium, Enterococcus, Ruminococcus and Roseburia6.
An indirect demonstration of the gut’s perimeter of action comes from lactate metabolism, generated by muscles during exercise. Lactate not only re-enters the liver to initiate gluco-neogenesis, it also enters the intestinal lumen via blood circulation. Here, it selects the Veillonella strain, causing the production of the SCFA propionate. As a recent study demonstrates7, this simple fact considerably enhances physical capacity; so it can be stated that microbiota-sourced SCFAs improve performance.
Happiness, as well as performance, seems to depend on the chain of events beginning with food intake — the selection of goal-oriented microbial strains, synthesis of SCFA byproducts, and the influence of these on the brain8.
Moreover, the liver is also a site of integration for the link between the immune system and the brain9. Of note, the potential communication pathways between the liver and the brain are woven into a complex network whereby the liver is innervated by vagal afferents that can respond to immune mediators, such as TNF-α, IL-1, and IL-6. Vagal afferents then project to the dorsal vagal complex (the nucleus tractus solitarius (NTS), area postrema, and dorsal motor nucleus). From there, they then reach a variety of cerebral regions, including the para-ventricular nucleus of the hypothalamus.
Pro-inflammatory cytokines, associated with a diversity of appalling processes, either physical or psychological, can interact with their receptors on cerebral endothelial cells (CEC) to induce their respective signalling pathways. Therefore, circulating TNF-α, IL-1 and IL-6 can access the brain, via the circumventricular organs (CVO) and the choroid plexus, regions of the brain that lack a tight blood-brain barrier. Activated monocytes will at that point be able to transmigrate into the brain in response to the instigation of resident cerebral microglia cells via a potent monocyte chemoattractant (MCP-1). These liver-to-brain communication pathways will result in changes in central neural activity and, thereby, behavioural alterations. One of the most deleterious effects of dysbiosis, the ‘sickness disease’ generated by gut barrier dysfunction and primarily associated with these liver-brain routes will be described later. In fact, inflammatory elements are always around, essentially due to the inability to cope with stress. At the same time, a general disregard for the available resources to obtain efficient anti-inflammation, that is the important effects of vagus stimulation on cell reprogramming, barrier renewal and anti-inflammatory activity, seems widespread among physicians as well as their patients.
Help is coming from the immune system, where some essential regulators have been selected throughout evolution to avoid any degradation that goes too far or too fast.
Intestinal cells and the immune system
Intestinal stem cells are involved in continuous self-renewal and differentiate into the specialised cells of the intestinal epithelium. Accordingly, an important quality control mechanism of the gut barrier is performed by intra-epithelial lymphocytes (IELs) and aromatic hydrocarbon receptors (AhR) as supervisors organised at the level of the waterproof and flexible epithelial barrier. In the absence of AhR, IELs disappear and epithelial growth decreases, favouring inflammation10. Cruciferae (such as broccoli or brussel sprouts) in particular favour the development and maintenance of this unique house-keeping mechanism. The latter controls the functional selection of enterocyte quality, produced continuously by the crypts of intestinal villi of the adult intestine. Signatures of mutational processes, such as in pre-cancerous cells, can hence lead to cell destruction and the prevention of cancer11.
If the intertwined relationship between food and microbiota is becoming better understood, it can be partly attributed to the fact that the bacterial metabolism of dietary fibres impacts epigenetics. SCFA and folates, produced following the consumption of phytochemicals, have indeed been revealed to be epigenetic modifiers12. Could the microbiota also induce epigenetic modifications associated with ageing? Global hypo-methylation and local hyper-methylation, characteristically found in ageing, could perhaps become avoidable if DNA reading processes could take into consideration the quality of food intake.
Inflammation
In the second part of this article, the origin of inflammation will be considered. Indeed, why can it be said that chronic inflammation comes from chronic stress? And, according to the above, what could be the beneficial anti-inflammatory and anti-stress role of stimulating the vagus system?
Apart from the help we can seek through appropriate nutrients selecting advantageously strategic strains of our gut bacteria, the vagus nerve indeed joins in contributing to generalised anti-inflammation. Being the anatomical structure connecting the gut and the brain via specific signalling initiated (but not only) by food intake, it deploys a general function of orchestrating the ongoing exchanges between gut and brain13.
Brain and stress management
Within our whole body, each organ, as well as each millimetre of our skin, is equipped to treat the constant challenge of life — stress. The brain, in particular, has developed a quite complex system to treat incoming signals brought by stressful events. Following a stress impulse, a dialogue between the two opposite parts of the brain begins, respectively, in the pre-frontal cortex area with its more regulatory and mindfulness oriented approaches and the limbic system hosting the emotional components. The resulting input to the mesolimbic area then drives two important actions. The first is to minimise the shock effect of stress, through the secretion of dopamine, aiming at re-establishing motivation and pleasure. This deeply influences behaviour, brought by the strong emotions of wanting, craving, mating. The second and less known aspect of this compensatory strategy consists of the registration of the assumed behaviour by the basal ganglia that will, in case of future stresses, immediately be re-adopted. A habit has been created14.

Moreover, during a stressful event, the brain also triggers the adrenal glands to produce cortisol15. Because of the potent anti-inflammatory effects of this hormone, people normally do not even realise they are under stress. The distinction should be mentioned here between acute and chronic stress. Acute stress is reversible and involves a whole cohort of nerve fibres, neuropeptides, mast cells, kinins, prostaglandins, histamine, serotonin, leukotrienes, and PAF acether. Endogenous or exogenous anti-microbial agents (lactoferrin, IgA, transferrin, lysozyme, defensins) also populate this landscape16. Finally, acute inflammation also induces the production of pyrogenic cytokines such as IL-1, TNF-α, IL-6 and IFN-γ as well as their opponents, the natural cryogens arginine, vasopressin, α-MSH, glucocorticoids, Neuro-Peptide-Y (NPY), bombesin, thyroliberin and IL-1Rα. In fact, acute inflammation is of great benefit for the body. Usually, tissue self-repair is accomplished via functional stem cell-like cells17. As acute inflammation is the depositary of archetypal pathways, such as that of nuclear factor-κB (NF-κB), it also promotes an open configuration of the chromatin, for the greatest possible epigenetic plasticity. The important positive actions of acute inflammation have been recently analysed from a more functional perspective. The newly coined term of ‘transflammation’17,18 refers to the benefit of plasticity, allowing increased epigenetic flexibility as well as phenotypic malleability to adapt responses to stress, injury and disease. At this stage, inflammation is our first adapting tool to the environment. The story is different with chronic inflammation.
Chronic inflammation
Life in our modern society is characterised less by acute stress when compared to ancient times. Chronic stress has become the rule, so that the chronic inflammation that ensues is now considered the origin of many diseases, such as depression, hypertension, cancer, and neurodegenerative diseases. In a fascinating way, the same mechanisms that try to respond to traumatic or infectious stress are at work during emotional or psychological stress. But only long-lasting, insidious stress, obsessive thoughts and great psychological pains, such as mourning, are able to install and maintain a vicious cycle. Then, as soon as stress becomes chronic, chronic inflammation appears.
Classic signs of chronic inflammation are those affecting an individual’s quality of sleep — such as difficulty falling asleep — as well as mood, with negative or obsessive thoughts. Difficulty concentrating and the appearance of pain without any identifiable underlying pathology are other hallmarks of chronic stress associated with chronic inflammation. Finally, the reluctance to meet others, asociality and isolation appear and inflammation is no longer a useful tool for adapting to our environment.
Even more insidiously, major depressive disease (MDD) has been identified within governmental institutions and international enterprises, as a result of a line of management exercising unsustainable pressure on employees19. According to the WHO20, in 2030 the second highest cause of death worldwide is foreseen not to be infectious diseases, cardiovascular disorders nor cancer, but premature death due to overdosage of legal antalgics such as codeine, regularly prescribed by physicians. The new scenario of chronic inflammation will thus be ‘mental illness in the workplace’ with its disability, absenteeism and sickness benefits.
But why should the gut be concerned with stress and chronic inflammation, the brain being in charge of stress-coping mechanisms? The link between the brain and gut in stress management and its consequences for the whole body is represented by cortisol. In fact, endogenous stress-coping mechanisms generate generalised inflammation21. Several conditions, such as pregnancy or menopause, dietary proteins and sugars, antibiotics and infections, toxins and food allergens and of course, stress, have all been described to challenge the intestinal barrier. However, most of all, it is cortisol that impacts the gut barrier. The condition of barrier dysfunction due to mechanical disruption of the tight junctions between enterocytes is called ‘leaky gut’ or dysbiosis. It can be easily measured by assessing zonulin levels22, as this molecule is the only physiologic modulator of intercellular tight junctions (ranges being >78 ng/mL in faeces and > 48 ng/mL in serum).
However, barrier dysfunction does not only compromise tolerance in the gut, impeaching the true anti-inflammatory role of mucosal immunity. It is also at the source of the so-called ‘sickness disease’23 that curiously carries all the symptoms of chronic inflammation, now exported from the gut to the whole body. Therefore, loss of memory, anxiety, refusal to meet at social events, obsessional thoughts, difficulty to concentrate, depression, chronic fatigue syndrome, sleeplessness, loss of interest, and obsessional neurosis are signs both of chronic inflammation and a sickness disease exported through the whole body.
How can the gut microbiota re‑establish the balance?
As mentioned above, food choices have an influence on gut de-inflammation by selecting the beneficial bacterial strains of our microbiota, mostly those generating SCFA. Indeed, the ingestion of cellulose fibres (pre-biotics), as well as probiotics (yoghurt, cheese), favours the growth of beneficial bacterial strains such as Bifidobacteria, Clostridium, Enterobacterium, Enterococcus, Ruminococcus, and Roseburia.
Pre- and pro-biotics have been dubbed ‘psychobiota’24 because their combined action has been shown to reverse anxiety and depression in animal and human studies25.
Moreover, the microbiota has neuroactive potential that is best identified within bacterial strains of the Faecalibacterium and Coprococcus genus. The amount of these producers of propio-butyrate is considered an indicator of good QoL. Together with Dialister and Coprococcus spp., their levels decrease in cases of depression, even after correction for the confounding effects of antidepressants26.
Therefore QoL seems to be dependent on bacterial strains of the microflora. Scores for mental parameters, such as mental health (MCS), emotional well-being (MH), social functioning (SF), vitality (VT), as well as physical parameters, such as general perception (GH), physical functions (PF), role limitations (RP), burden pain (BP), and physical compliance score (PCS) can vary following bacterial activity27.
In summary, challenged by stress, the brain sets up rescue mechanisms, with essential motivation strategies (dopamine, reward, compensating behaviour) on the one hand and the transient control of inflammation via cortisol secretion by adrenal glands on the other. Unfortunately, the vicious cycle of reward may promote addictive behaviour and the anti-inflammatory action of cortisol, initially beneficial, can become fatal for the gut barrier function in the long run. In fact, the complex sickness syndrome that is brought on is due to neuro-inflammation of the brain as a consequence of the generalised inflammation and its exportation from the gut to the whole body. With its ensuing chronic fatigue syndrome, loss of memory, sleeplessness and depression, the general impression is that of the beginning of a neuro-degenerative disease, yet ‘only’ chronic inflammation and dysbiosis are operating.
The vagus stimulation maintains skin quality
Why is the brain-gut connection relevant to skin beauty and ageing? Should we care about systemic inflammation to improve skin quality? Should we be feeding it with special ingredients? What should we eat to stay young? Or should we be fasting? Indeed, intense gut and skin renewal after periodic fasting have been described28. Today we know that to improve the health and quality of the skin, we can compensate salty/sweet craving with nutraceutical supplementation, cosmeceutics and specific pre-biotics29.
Beside the gut-brain connection and its global effects on the body, the skin displays a fully functional peripheral hypothalamic–pituitary–adrenal (HPA) system around the hair follicle30. These ‘micro-brains’ sense stress, such as that induced by infection, exsiccation, heat/cold, UV light, ozone, toxic agents, stretch, mechanical damage, free radicals, allergens, and high/low humidity. Moreover, psychological stress is able to stop the proliferation of melanocytes via corticotropin-releasing hormone (CRH) receptor31 activation, while accelerating that of keratinocytes. Likewise, odorant molecules are able to induce potent repair activities, activating the pool of progenitor cells around the sebaceous glands woven within the structure of the hair follicle32.
With the gut-brain axis being the major source of systemic inflammation, compromising microbiota-driven anti-inflammatory strategies, the only anti-inflammatory and deeply repairing system that remains available for the restoration of health and beauty of the skin is the activation of the vagal system. Stimulating the vagus nerve involves quite trivial actions, such as indulging in a taste you like, or receiving an affectionate skin touch from a friend. Social grooming, watching a healing image, controlled breathing consistent with cardiac coherence or even listening to enchanting sounds all potently induce vagal activity. These activities, which can be summarised by the term of ‘pleasures’, activate the vagal system, induce relaxation and repair, accompanied by a decrease of all biochemical markers of inflammation33.
In summary, the gut-brain connection influences skin quality through three interconnected mechanisms:
- Stress can destabilise the gut barrier function, thus exporting the inflammatory reaction to the whole body
- Chronic inflammation fuels ageing
- Nutrition may become a potent inductor of epigenetic modifications
- The gut-brain connection can also ameliorate skin quality when solutions from natural substances are taken into account that would operate for barrier restoration34 and treatment of systemic (chronic) inflammation.
In the European study RISTOMED35, healthy human volunteers submitted to a controlled diet plus various supplementations. The anti-inflammatory effect of the latter was shown, for d-Limonene supplementation, by a decrease in circulating IL-6 levels, associated with the amelioration of CES-D scores (Center for Epidemiological Studies-Depression Score). In another study, testing the same nutritional supplementation, psoriasis depending on stress-induced dysbiosis and chronic inflammation responded similarly with the disappearance of redness, itching, and increase of QoL36.
Therefore, a four-point conundrum focusing on gut barrier restoration, vagal mood regulation and repair activity, resulting in inflammation control should be surveyed when skin health and beauty are the focus, eventually addressed by bioactive compounds37,38.
In conclusion, when chronic inflammation invades the digestive system as a consequence of the brain’s anti-inflammatory strategy via cortisol, affecting the gut barrier function, a starting point of a generalised alteration involving all organs is reached. Vice-versa, the rescue could come from gut microbiota, linked to the possibility to promote repair, anti-inflammation, cancer prevention and healthy living.
Declaration of interest None
Figures 1 © Dr d’Alessio
REFERENCES
- Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701-712
- Cheyne G, The natural method of cureing the diseases of the body and the disorders of the mind, (1742), pp. 295-296
- den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013; 54: 2325–2340
- Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010;140:859-870
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804-810
- Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol. 2012;10:323-335
- Scheiman J, Luber JM, Chavkin TA, MacDonald T, Tung A, Pham LD, et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med. 2019;25:1104-1109
- Li B, Selmi C, Tang R, Gershwin ME, Ma X. The microbiome and autoimmunity: a paradigm from the gut-liver axis. Cell Mol Immunol. 2018; 15: 595-609
- D’Mello C, Swain MG. Liver-brain inflammation axis. Am J Physiol Gastrointest Liver Physiol. 2011;301:G749-761
- Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629-640
- Lee-Six H, Olafsson S, Ellis P, Osborne RJ, Sanders MA, Moore L, et al. The landscape of somatic mutation in normal colorectal epithelial cells. Nature. 2019;574:532-537
- Paul B, Barnes S, Demark-Wahnefried W, Morrow C, Salvador C, Skibola C, et al. Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clin Epigenetics. 2015; 7: 112
- Tilg H, Moschen AR. Food, immunity, and the microbiome. Gastroenterology. 2015;148:1107-1119
- Epel, E. S., Tomiyama, A. J., Dallman, M. F. (2012). Stress and reward: Neural networks, eating, and obesity. In K. D. Brownell & M. S. Gold, Food and addiction: A comprehensive handbook (p. 266–272). Oxford University Press
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185
- Coates M, Lee MJ, Norton D, MacLeod AS. The skin and intestinal microbiota and their specific innate immune systems. Front Immunol. 2019; 10: 2950
- Menendez JA, Alarcón T. Senescence-inflammatory regulation of reparative cellular reprogramming in aging and cancer. Front Cell Dev Biol. 2017; 5: 49
- Meng S, Chanda P, Thandavarayan RA, Cooke JP. Transflammation: Innate immune signaling in nuclear reprogramming. Adv Drug Deliv Rev. 2017; 120: 133-141
- Woo JM, Postolache TT. The impact of work environment on mood disorders and suicide: evidence and implications. Int J Disabil Hum Dev. 2008;7:185-200
- Csete J, Kamarulzaman A, Kazatchkine M, Altice F, Balicki M, Buxton J, et al. Public health and international drug policy. Lancet. 2016;387:1427-1480
- Clarke G, Sandhu KV, Griffin BT, Dinan TG, Cryan JF, Hyland NP. Gut reactions: breaking down xenobiotic-microbiome interactions. Pharmacol Rev. 2019;71:198-224
- Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91:151-175
- Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am. 2009;29:247-264
- Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016;39:763-781
- Bourassa MW, Alim I, Bultman SJ, Ratan RR. Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56-63
- Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol. 2019;4:623-632
- Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735-742
- Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 2015;22:86-99
- Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med. 2017;9(378):eaah4680
- Paus R. Exploring the “brain-skin connection”: Leads and lessons from the hair follicle. Curr Res Transl Med. 2016;64:207-214
- Slominski A, Zbytek B, Zmijewski M, Slominski RM, Kauser S, Wortsman J et al. Corticotropin releasing hormone and the skin. Front Biosci. 2006;11:2230-2248
- Busse D, Kudella P, Grüning NM, Gisselmann G, Ständer S, Luger T, et al. A synthetic sandalwood odorant induces wound-healing processes in human keratinocytes via the olfactory receptor OR2AT4. J Invest Dermatol. 2014;134:2823-2832
- Reardon C. Neuro-immune interactions in the cholinergic anti-inflammatory reflex. Immunol Lett. 2016; 178: 92-96
- d’Alessio PA, Ostan R, Bisson JF, Schulzke JD, Ursini MV, Béné MC. Oral administration of d-limonene controls inflammation in rat colitis and displays anti-inflammatory properties as diet supplementation in humans. Life Sci. 2013;92:1151-1156
- Ostan R, Béné MC, Spazzafumo L, Pinto A, Donini LM, Pryen F, et al. Impact of diet and nutraceutical supplementation on inflammation in elderly people. Results from the RISTOMED study, an open-label randomized control trial. Clin Nutr. 2016;35:812-818
- Rossi A, Béné, MC, Carlesimo M, Garelli V, Fortuna MC, d’Alessio PA. Clinical efficacy of orange peel extract in psoriasis. Global J Dermatol Venerol. 2015; 3: 1-4
- d’Alessio P, Ostan R, Valentini L, Bourdel-Marchasson I, Pinto A, Buccolini F, et al. Gender differences in response to dietary supplementation by orange peel extract in elderly people in the RISTOMED study: impact on quality of life and inflammation PRIME 2012;July/August:30-37
- d’Alessio PA, Menut C, Lejay J, Bisson JF, Béné MC. Anti-inflammatory and skin repair treatments with d-Limonene. PRIME. November 2015; 19-25