During menopause, the hormonal metabolism undergoes significant changes, leading to some typically unpleasant symptoms such as hot flushes and anxiety, and leading to an increase in the relative risk of cardiovascular disease, osteoporosis and declined cognition. In order to overcome these symptoms, hormone replacement therapy (HRT) is usually prescribed. As recent data has shown the association between HRT and the risk of breast cancer, the routine prescription of HRT has become a significant concern for both patients and physicians. Despite an ongoing debate between conventional HRT and bioidentical HRT (BHRT) users, the purpose of this article is to show that the risk of breast cancer is a result of patients’ individual oestrogen metabolism, and that personalisation is the key to increased safety when using HRT. Therefore, before prescribing any HRT, including BHRT, an assessment of the patient’s genetic make-up for the production of dangerous oestrogen metabolites, and an identification of her oestrogen load from endogenous oestrogens, environmental oestrogens, and dietary oestrogens should be undertaken. This will allow the physician to estimate the most adapted hormonal dosage, as well as evaluate whether the beneficial effect of HRT will outbalance the potential risk of developing breast cancer. Treatment with BHRT then becomes personalised and safe. In order to increase safety and — in addition to the adapted hormonal dosage — personalised nutritional modulation of oestrogen metabolism is prescribed knowing the specific single nucleotide polymorphism of the genes involved in oestrogen metabolism.
Breast cancer is the most common cause of cancer mortality in women worldwide, and its incidence continues to increase1. The majority of breast cancer cases (90–95%) are sporadic (i.e. non-hereditary), with only a modest or absent family history of breast or ovarian cancer. Sporadic cancer is considered to be a polygenic or multifactorial disorder involving many genes that interact with nutrition and lifestyle factors2. In addition, oestrogen exposure is one of the most important risk factors as its metabolites can attack DNA and cause double-stranded breaks. For this reason, the functional polymorphisms of genes involved in oestrogen metabolism should be identified for inclusion in a multi-gene breast cancer risk reduction approach, as well as prior to the commencement of any hormone replacement therapy (HRT).
The dangers of hormone replacement therapy
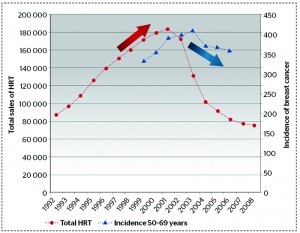
Conventional HRT has been used for many years without any major health concerns, until The Women’s Health Initiative randomised controlled trial (which abruptly ended in July 2002) found that the overall health risk exceeded the benefit from the use of combined oestrogen and progestin for an average 5.2-year follow-up among healthy postmenopausal women3. The study found a 26% increase in breast cancer cases, as well as increases in cardiovascular disease, deep vein thrombosis and stroke. Similarly, the Million Women Study found that the use of HRT by women aged 50–60 years in the UK over the previous decade resulted in an estimated 20000 extra breast cancer cases, 15000 of which have been associated with oestrogen–progestin4. It was found that conjugated equine oestrogens (CEE) and synthetic progestins in a conventional HRT regimen prescribed to every woman in the same dose, was mostly to blame (Figure 1).
However, the French E3N cohort study showed an unequal risk for breast cancer associated with different HRT regimens (conventional versus bioidentical), giving a better idea as to the direction in which HRT treatment approaches should go5 (Figure 2). From this study, it is possible to surmise that the smallest relative risk for breast cancer is after the use of combined oestrogen–progesterone, preferably not longer than 6 years, and in combination with weaker oestrogens (e.g. estriol).