Key points to consider when using PRP
There are great differences among the commercially-available PRP preparations that the aesthetic practitioner should be aware of, including18,19:
- The volume of autologous blood required, which can vary depending on the kit used
- The time and speed of centrifugation
- The nature of plasmapharesis, including the removal, treatment, and return of (components of) blood plasma from blood circulation
- The addition of an activating agent and its effect on pH
- The final platelet, growth factor, and leukocyte concentrations.
Weibrich et al20 looked at platelet counts as well as different growth factors (PDGF, TGF-β-1, IGF-1), and found that there was substantial variation in the growth factor content of PRP. They concluded that the factors influencing this are still worthy of further investigation and that a technique whereby the growth factor content could be rapidly assessed in PRP may be of therapeutic benefit. The platelet, and consequently the growth factor, availability also depends on the use of the harvesting kit.
Mazzucco et al21 found that, ‘Similar methods for platelet gel preparation revealed different performances concerning growth factor recovery and the kinetics of its release from the gel. It is unclear whether these noticeable differences are important for clinical management.’
Further features of PRP, such as the amound of blood that is needed, can be seen in Table 122. In fact, it is often considered that the lowest draw of blood is actually more beneficial for treatment, as well as a shorter time of centrifugation, which maintains the platelet’s integrity and practicality. The physiological pH means that when the PRP is injected, cellular integrity is further maintained, and the patient will experience only a minimal sensation of pain. It is also important to be wary of potential contamination and the resulting purity of the final PRP product.
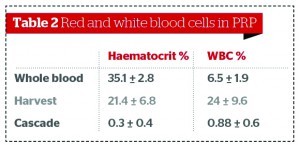
The purity of the final PRP product matters. When high numbers of red blood cells are present in the final PRP formulation, the erythrocytes present will induce the oxidisation of extracellular haemoglobin, thus releasing heme (the deep red, non-protein, ferrous component of haemoglobin, C34H32FeN4O4) and reactive ferric oxide23,24. Free radicals are generated, which lead to inflammation, vascular injury, and cell death; additionally, free heme can lead to haemosiderin staining from iron oxide25.
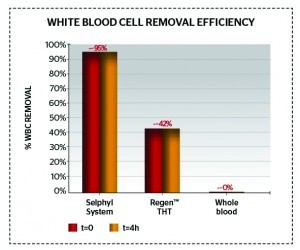
Figure 2 Effective removal of white blood cells from PRP (only 0.62 ± 0.22 white blood cells 103/mm3)
Some authors do not highlight the controversial issue of whether high or low concentrations of white blood cells in the final PRP are desirable or not. If high numbers of white blood cells are present in PRP, leukocytes will also be present, which release matrix metallo-proteinases (MMPs) and produce oxygen radicals/cytokines (catabolic or inflammatory26). Thrombin also significanly increases leukocyte IL-1β production18, which can lead to tissue damage, inflammation, and impaired healing as well as localised pain26. As a result, it is desirable to have low volumes of white and red blood cells in the final PRP product, as well as low and consistent rates of platelets, to ensure PRP quality. However, authors such as Kawazoe T et al26 do state that PRP enhanced with white blood cells leads to more tissue augmentation than PRP alone.
Figures 2–3 and Table 2 show comparative data on the quantities of red and white blood cells in final PRP products22,28.
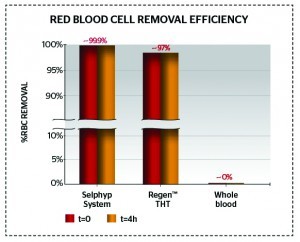
Figure 3 Red blood cell removal showing a 25-times greater purity (only 0.2% red blood cell contamination)
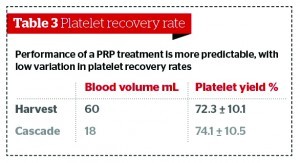
It is also necessary to consider the concentration of platelets in PRP preparations, and what the optimum is for treatment. There is evidence that, in humans, for certain applications such as bone regeneration, low concentrations of platelets are more beneficial than higher concentrations18. Studies have found that platelet count significantly impacts on the variation in growth factor bioavailability29, with some suggesting up to a 27-fold difference21. Furthermore, the performance of the final PRP preparation can be less predictable as a result, with an increasing variation in platelet recovery efficiency. Table 3 shows that the performance of a PRP treatment is more predictable, with a low variation in platelet recovery rates.
Activation of PRP formulations
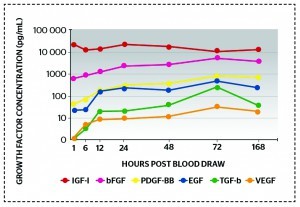
Figure 4 Controlled growth factor release over time: five- to ten-times increase in each growth factor indicates increased stability within the PRFM over 7 days
When administering a PRP treatment, a number of endogenous and exogenous factors are activated:
- Endogenous Dermal collagen and exposed endothelial collagen, arachidonic acid (inflammation pathway), thromboxane A2 (inflammation pathway), adenosine diphosphate (ADP), thrombin, substrate bound ligands of glycoprotein IIa/IIIb, vasopressin, and adrenalin
- Exogenous Adrenalin, CaCl2, controlled heat, vibration/spinning, thrombin (bovine).
These activators cause the formation of a fibrin clot, or the release of growth factors from platelets14–16. Calcium chloride further impacts on a controlled activation process; a controlled fibrin formation is desirable, and an exact amount of calcium chloride will produce a fibrin matrix. The combination of PRP with CaCl2 — in a defined ratio — leads to the formation of PRFM to scaffold thrombocytes, avoid their premature activation and degranulation, enable a sustained growth factor release, and the migration of stem cells and reservoir for regenerative signalling14–16. Figure 4 shows that there is a controlled release of growth factors over time, indicating increased stability in the PRFM over 7 days. This further supports the evidence that the quality of different PRP preparations is very different, and that the physician should be cognisant of such differences when choosing a kit with which to harvest the PRP. The building of a fibrin clot (after injection in vivo) means that the platelets are embedded in the injection area to create a scaffold formation, consequently impacting on a sustained growth factor release following treatment.
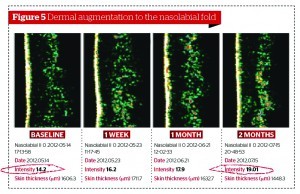
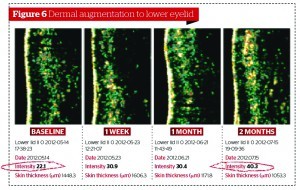
Scalfani et al30, using the injection of PRFM, aimed to induce collagenesis, angiogenesis, and adipogenesis on upper arm histologies. In order to do this, at 7 days post‑treatment, fibroblasts had been activated and new collagen deposition had appeared. At 19 days post‑treatment, the effects had increased greater still, with the formation of new blood vessels and stimulation sub-dermal adipocytes. Likewise, Cho et al31 found that the appearance of wrinkles in the PRP‑injected group of their study were significantly reduced and dermal thickness was improved.