Adele Sparavigna, MD discusses how practitioners can counteract the signs of ageing in the periorbital area

Eye contour aesthetics represent a matter of great concern since perceptions regarding the degree of a person’s fatigue and age are primarily based on the periorbital area(1,2). Throughout cultures, societal attitudes are similar in that periorbital dark circles, bags and wrinkles contribute to a tired, aged and even unwell appearance. Nowadays, an important effort, both in terms of eyelid surgery and soft tissue filler injections, is being conducted to counteract eye-contour problems. Soft tissue filler injection is the second most common minimally invasive procedure performed with an increase year over year. A substantial percentage of these injections is used to treat the tear-through.
Dark circles play a central role in the appearance of the ageing and fatigued lower eyelid and are very difficult to erase completely. Both injectables and injection techniques are of fundamental importance for a successful treatment. The quality of the infraorbital skin strongly contributes to the ageing appearance of this area. Lower eyelid skin is very thin and allows subdermal features to be visible on the surface. At this level, when minimal subcutaneous tissue is represented, the skin has an intimate relationship with the orbicularis muscle and vessels. This results, with age, in changes that are more exaggerated compared with the rest of the facial skin. The thin and somewhat transparent eyelid skin provides little camouflage to the prominence of the underlying midface soft tissue, including the robust subcutaneous vascular network and the orbicularis oculi muscle. This results in a darkened appearance of the skin. Furthermore, fluids may accumulate in the periorbital soft tissue area giving rise to eyelid swelling, the so-called bags.
Infraorbital bags can be augmented by the protrusion of fat from the orbital cavity. Age-related anatomic changes in soft tissue include subcutaneous fat atrophy and volume loss in the malar area, hypertrophy of orbicularis oculi muscle, pseudoherniation of sub-orbicularis oculi fibro-adipose tissue.
Injectables for dark circles and bags
Peculiar skin and anatomy issues in the periorbital area deserve special injectables(3). Although cross-linked hyaluronic acid (HA) fillers have become the treatment of choice in many settings to address under-eye contour irregularities, using a common filler can be very complicated in this region and may give rise to accumulation of not properly injected product. Extra Cellular Matrix (ECM) targeting is a real advanced solution in the treatment of the periorbital area since it is based on skin physiological bio-regeneration by stimulating the production of new collagen and elastin fibres together with HA. The effects are twofold: the sunken region is lifted and the skin is stimulated to produce more collagen and elastin, giving a re-volumizing result to the area around the eye. SUNEKOS® 200 injectable (by Professional Dietetics, Milan, Italy) is an ECM targeting treatment containing low molecular HA and a patented amino acids mixture capable to physiologically promote new collagen and elastin synthesis into the injected area. Tolerance and aesthetic performance of this product on the area around the eyes is particularly expected based on the extreme fluidity and proper composition of the formula.
Anatomy and injection techniques
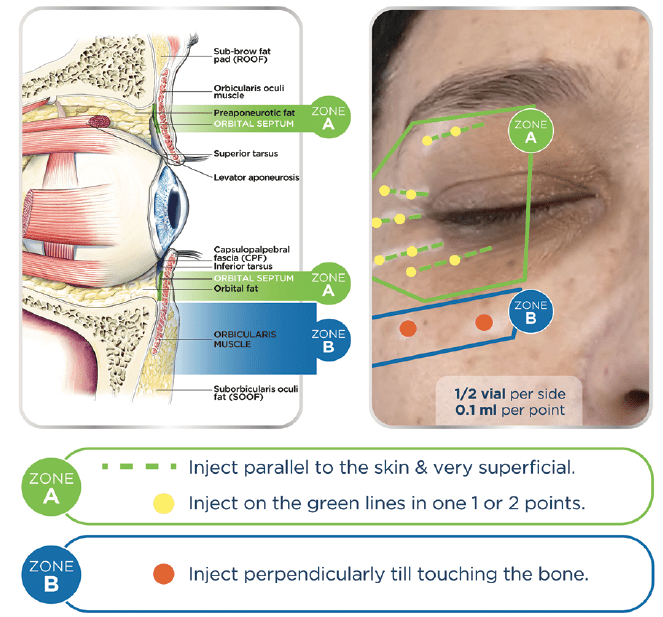
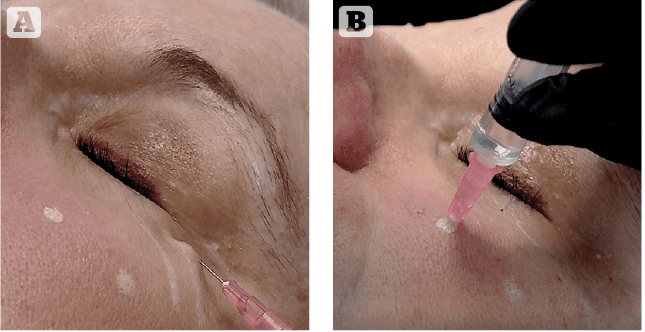
Since periorbital skin is a difficult area to treat, a detailed understanding of facial anatomy is crucial to inject this area properly (Figure 1). Both eyelids can be divided into two parts: mobile and fixed. The mobile part of the skin is very thin and has to be injected very superficially since it lies on the orbital septum (Zone A on Figure 1). Just over the orbital septum, there is fat tissue that has low vascularization. Any injected substance in this area results in accumulation and is eluted by the bloodstream with difficulty. The fixed part of the eyelid lies on the orbital septum, fat tissue, muscle and bone. Age-related changes in the midface result in relative orbital rim recession and midfacial and malar bone volume loss, leading to a tightening of the orbital and facial retaining ligaments. As facial fat descends and fat volume decreases, the relatively inflexible ligaments result in tethering and associated orbital rim and facial hollowing. These hollows lead to a worsening of shadowing, which can particularly be noted in the tear trough area in the inferomedial orbit. The proposed injection technique takes into account the difference between the mobile part and the fixed part of eyelids. The mobile part is injected using a superficial retrograde linear technique (Figure 2A). A 30G needle has to be inserted parallel to the skin surface and must be visible through the skin while releasing a small amount of the product. The fixed part is injected, releasing a bolus of 0.2 ml directly on the underlying bone surface. A 30G needle has to be inserted perpendicular to the skin surface to hit the bone and release the product (Figure 2B).
Aim of the study
The primary endpoint of this study was to evaluate clinically and through non-invasive instrumental measurements the aesthetic performance on the area around the eyes with the SUNEKOS® 200 injectable treatment. In particular, three micro-injection sessions with an interval of 15 days were performed by a specialized dermatologist using periosteal and subepidermal injection techniques. This injective procedure involves a micro-wheal of 0.2 ml just above the periosteum and micro-wheals of 0.1 ml very superficially, just under the epidermis.
The amount of product to be injected was 3 ml (1 vial) for each injective session (1.5 ml for each side of the face).
All evaluations were carried out mono-laterally at:
- Baseline (T0)
- T3+1d — 1 day after the last injection treatment with Sunekos® 200
- T2M — 2 months from the first injection procedure
- T3M — 3 months from the first injection procedure.
- Five visits were provided during the trial: a basal visit, two intermediate visits and two follow-up visits.
Product composition
SUNEKOS ® 200 is a CE Class III medical device composed of small bottles containing sterile and apyrogenous lyophilized amino acids of Glycine, L-Proline, L-Leucine, L-Lysine HCL, L-Valine, L-Alanine and sterile vials containing sodium hyaluronate with low molecular weight, manufactured and distributed by Professional Dietetics S.p.A., used as a dermal biorivitalizer for the correction of photo-ageing and facial-ageing. This composition has an international patent for the formula for the production of elastin and collagen.
Clinical evaluations
Clinical assessment(4) was performed as follows:
A clinical grade assigned to the area around the eyes (Crow’s feet)
A visual score of crow’s feet was performed according to an internal reference photographic scale (visual score from 0 — no wrinkle, to 7 — very marked wrinkles). Dark circles and infraorbital bags were evaluated according to a visual score from 0 (absent) to 4 (very marked).
Non-invasive instrumental evaluation(5,6)
Wrinkle profilometry (Primos compact portable device, GFMesstechnik, Germany). Skin colour evaluation of the periocular area by optical colourimetry (Chroma Meter CR-200®).
Study population
The study was conducted on 22 female volunteers, aged 44–65 years (mean = 53), with dark circles and infraorbital bags. Informed consent had been obtained.
Results
The percentage variation versus T0 obtained for each clinical evaluation, are summarized below and in Figures 5-7:
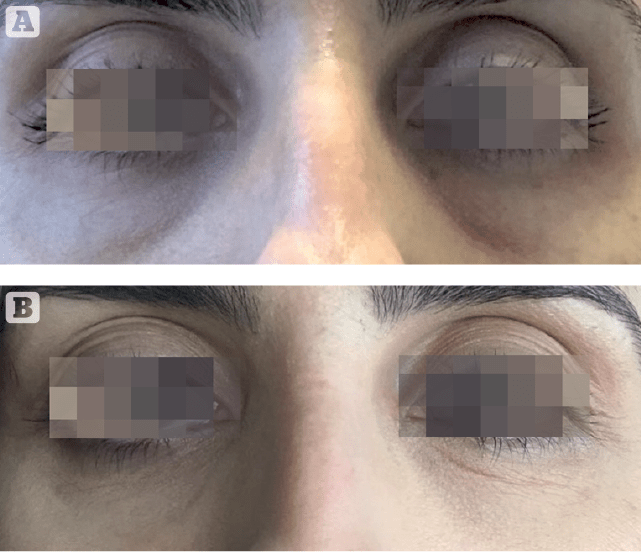
Only four subjects in the sample population were affected by infraorbital bags. Although no statistical evidence could be reached to properly evaluate infraorbital bags reduction, a trend toward an important improvement was observed (Figure 3).
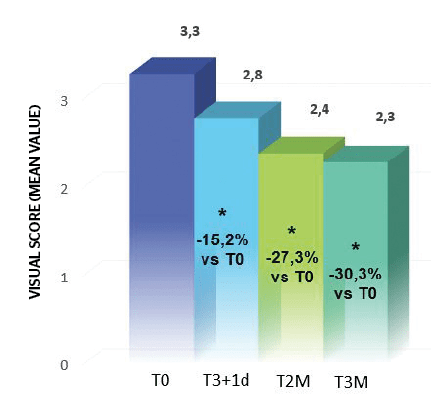
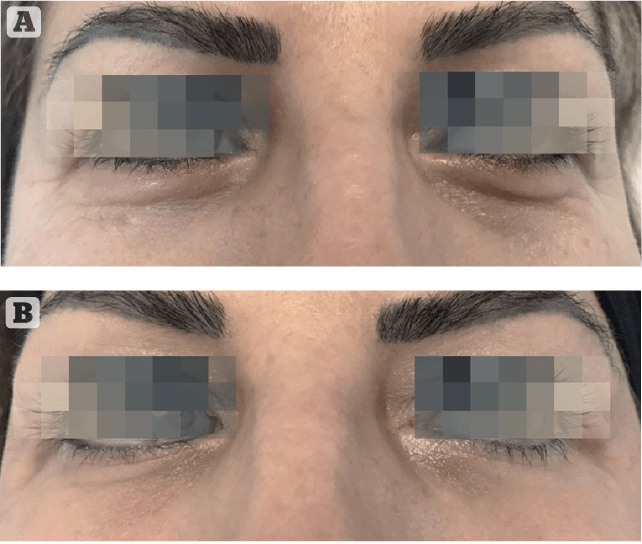
Obtained results highlighted an important reduction to the crow’s feet visual score (at least 1 grade) respectively in 48% of subjects at T3i+1d, 71% at T2M and 76% at T3M. Starting from T2M a significant reduction of dark circles (at least 1 grade in the visual score), 52% of subjects at T2M and 71% at T3M (Figure 4).
These results confirm treatment efficacy after the treatment and progressive improvement with time until 3 months after. They highlighted the aesthetic performance of the tested treatment on the area around the eyes.
Instrumental evaluations
Skin profilometry
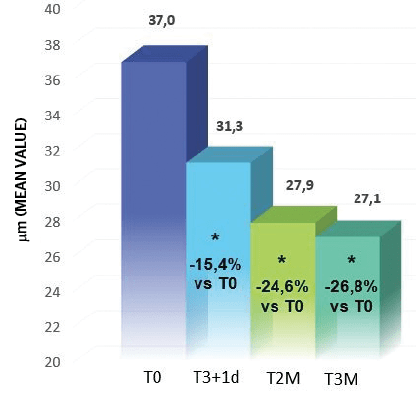
Image analysis of the area around the eyes (‘crow’s Feet’) confirmed the clinical assessment results; in fact, starting from T3i+1d an important and progressive ‘anti-wrinkle’ activity from the injectable treatment was demonstrated (Figure 5).
There was also a Ra (average roughness of the analysed profile) reduction of 15.4% at T3i+1d, 24.6% at T2M and 26.8% at T3M (Figure 6).
Skin colour measurement
The skin colour of the area around the eyes was evaluated by optical colourimetry7. The colouring of the dark circles is caused both by an accumulation of cutaneous chromophores and by vascular stasis; these conditions are difficult to distinguish because they can be contemporary and, in some way, linked.
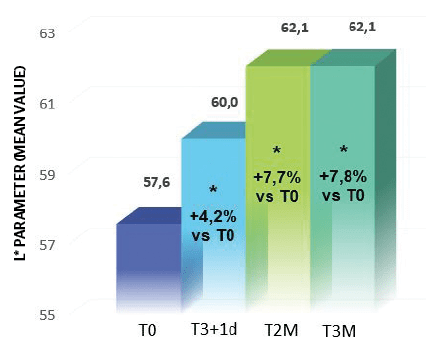
Results from the treatment highlighted a statistically significant increase of L* parameter, which indirectly represents skin brightness, starting from T3i+1d (Holm-Sidak Adjusted Wilcoxon signed-rank test p<0.05, T3i+1d, T2M and T3M vs T0); in particular the skin brightness improved 4.2% at T3i+1d, 7.7% at T2M and 7.8% at T3M (Figure 7).
Furthermore, it was demonstrated a clinically/statistically significant increase of a* parameter (skin redness) corresponding to 16.4% at T2M (Holm-Sidak Adjusted Wilcoxon signed-rank test p<0.05 vs T0) and 10.9% at T3M, index of a skin microcirculation improvement and a reduction of vascular stasis likely determined by the study product bio-revitalizing performance.

Efficacy evaluation by the volunteers
At the end of the trial (T3M), each volunteer filled in a questionnaire regarding treatment efficacy and tolerance.
Considering the sum of the medium, marked and very marked judgements, it’s evident the subjects’ positive judgement on the aesthetic performance of the study treatment.
Tolerance
A number of subjects displayed the appearance of light/moderate bruises on some injection points after one or more treatments, that resolved within 5–15 days; these reactions represent expected events imputable to the injection procedure, not related to the tested product, performed moreover in an area with high vascularization and a thin epidermis.
For these reasons, the investigator judged the product tolerance good to excellent in 100% of subjects as also confirmed by the subjects’ self-assessment.
Discussion and conclusions
Clinical and instrumental results clearly showed the important aesthetic performance of SUNEKOS® 200 injectable treatment on the area around the eyes.
In fact, considering the experimental design and the injection technique (periosteal and subepidermal injection techniques) adopted, the study determined a statistically significant reduction of the crow’s feet (lifting/anti-wrinkles effect) from the third injection procedure (T3i+1d); as well as an increase in skin brightness and improvement to skin colour (trophic effect) from 2 months after the first injection procedure (T2M).
Therefore, wrinkles around the eyes, dark circles and infraorbital bags appear generally less visible and marked.
The final product tolerance was judged good/excellent; in fact, no unexpected adverse reaction related to the injection procedures occurred during the trial. The most interesting result, in our opinion, is the progressive improvement of results over time (up to 3 months). This is the first clinical documentation of the use of a low molecular weight protocol with AA and natural HA(8,9) in an application context in which only cross-linked HA have been evaluated. Since cross-linked HA products are known to have a lower safety profile and biological activity, it is very interesting to have this new treatment method available.
Declaration of interest Derming received a grant from Professional Dietetics for conducting this research
Figure 1–2, 4–7 © Dr Adele Sparavigna; Figure 3 © Jana Schmitt-Gau
References
- Ascher B. et al. Full scope of effect of facial lipoatrophy: a framework of disease understanding. Dermatol Surg,. 32:1058-1069, 2006
- Farkas J.P. et al. The Science and Theory behind Facial Aging Plast. Reconstr. Surg. Glob. Open;1:e8; Published online 5 April 2013
- De Servi B., Orlandini A., Caviola E. and Meloni M. Amino acids and hyaluronic acid mixtures differentially regulate extra cellular matrix genes in cultured human fibroblast. J Biol. Regul. & Homeost. Agents, Vol. 32, no. 3, 517-527, 2018
- Monheit G.D. et al. A classification of facial wrinkles. Plast. Reconstr. Surg., 108:1735-50, 2001
- Hatzis J. Skin surface profile technique and its applications. Int J Cosm Sci, 13: 281-291, 1991
- Lemperle G. et al. Leveque JL. EEMCO guidance for the assessment of skin topography. J Eur Acad Derm Ven., 12: 103-114, 1999
- Serup J, Agner T. Colorimetric quantification of erythema – a comparison of two colorimeters (Lange Micro Color and Minolta Chroma Meter CR-200) with a clinical scoring scheme and laser-Doppler flowmetry. Clin. and Exper. Dermatology, July 1990; vol. 15, Issue 4, pages 267-272.
- Sparavigna A., Forte R. and Dioguardi F. S. Multicenter study for the evaluation of tolerance and efficacy of a new integrated aminoacid treatment on the aging face. J. of Plastic Dermatol., 3(3): 19-25, 2007
- Sparavigna A., Orlandini A. Efficacy and Tolerance of an Injectable Medical Device Containing Hyaluronic Acid and Amino acids: A Monocentric Six-Month Open-Label Evaluation. J Clin Trials volume 7, issue 4, 1-6, 2017