Overall skin rejuvenation, the elimination of fine lines and wrinkles, and ‘skin tightening’ to alleviate skin laxity have become extremely popular procedures in aesthetic and cosmetic practice, and have developed into an extremely patient-driven indication. A range of approaches using lasers, energy-based devices, and phototherapeutic sources have been reported over the past two decades with varying degrees of short- and long-term results, and patient satisfaction.
Lasers: ablative to nonablative and back again
Lasers have been applied for skin rejuvenation since the early 90s, and have worked through full face ablative approaches with CO2 and Er:YAG1–4, nonablative laser skin rejuvenation5, fractional nonablative laser rejuvenation6, and fractional ablative laser technologies7. Although the full face ablative approach remains the gold standard, patient downtime is very long and side-effects are severe, so the progression to the current nonablative and ablative fractional approaches were driven by patient desire for shorter downtime and mild sequelae. Despite these latter approaches gaining good results, many patients continue to look for a skin rejuvenation modality where downtime does not exist (‘the lunchtime procedure’) or is very minimal, but results are good. Treatment with radiofrequency (RF) energy was therefore explored as a possible answer.
Radiofrequency: from external to microneedle electrodes
Parallel with the development of the various laser approaches, and as a possible alternative minimally invasive indication to help alleviate the sequelae associated with full face resurfacing, RF energy was seen by some clinicians and researchers as another way to deliver coagulative damage to the dermis via an electrothermal reaction to obtain good results with minimal downtime. RF energy is simply electricity; however, at the normal 60 Hz frequency of household electricity, severe disruption of living tissues, especially the nervous system, can occur owing to exposure to the high-powered alternating current, often resulting in death. William T Bovie showed in 1926 that an electrical current at a high frequency of over 200 kHz could pass through human tissue without fatal consequences, and could indeed produce controlled electrothermally-mediated incision and coagulation with the electrosurgery knife which is still often referred to as ‘the Bovie’8. The frequency of the current used in the electrosurgery knife is part of the zone on the electromagnetic spectrum occupied by radio waves, hence the descriptor: ‘radiofrequency’. Centred on the RF principle, development of externally-delivered RF-based skin rejuvenation systems was triggered in the early part of the New Millennium9, and by the end of the first decade, had gained a great deal of popularity10.
Skin presents electrical resistance of varying degrees to an electric current, and Joule’s law tells us that when electrical energy meets resistance, heat is generated. The higher the resistance, or the higher the current, the greater the degree of heat; meaning electrothermal damage can be delivered to the skin by passing an RF current through it.
Firstly, electrothermal damage at the correct temperature range (usually from around 65°C) creates a zone of coagulation in which the tissue is dehydrated and slightly ‘cooked’ with immediate induction of skin shrinkage. This zone of electrocoagulative damage then kick-starts the wound healing process whereby an inflammatory response leads to neocollagenesis and neoelastinogenesis, which are in turn followed by the remodelling process, and which, in the ideal situation, replaces the original thermally-induced shrinkage with true tissue tightening.
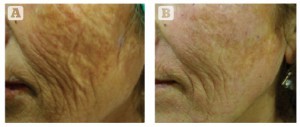
Figure 2 Severe photodamage and laxity in a 65-year-old Turkish female, Fitzpatrick skin type III. (A) Baseline findings. (B) 4 months after the final treatment session. Three MFR treatment sessions were given, 2 weeks apart, and three passes per session.
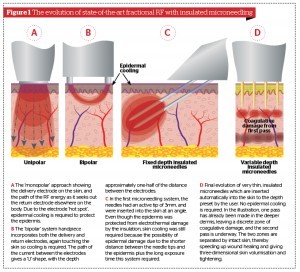
In RF systems, the energy is delivered via electrodes. Scientifically speaking, RF systems for skin rejuvenation all require at least one return and one delivery electrode, and are therefore bipolar. However RF devices were (and still are) popularly (but erroneously) classed by the method of delivery, namely ‘unipolar’ systems, ‘bipolar’ systems, and ‘multipolar’ systems where multiple delivery and return electrodes, usually in the form of small pins with or without plate electrodes, are incorporated in the handpiece. An additional problem is that the ‘hot spot’ for all external RF systems is at the electrode or electrodes, so when electrodes are in contact with the epidermis, aggressive skin cooling is required to prevent unwanted electrothermal damage to the epidermis, while still allowing the current to pass through into the target tissue in the dermis.
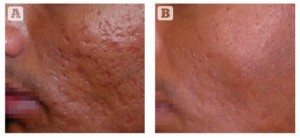
Figure 3 Acne scarring (rolling and box car type) on the cheek of a 37-year-old Indian male, Fitzpatrick skin type IV. (A) Baseline findings. (B) 1 month after a single MFR session, three passes (two passes over the whole face and one further pass over the scars).
Hantash and colleagues in 2009 presented an ideal solution to these double problems of reaching the ideal damage depth and epidermal preservation with microneedle delivery of RF energy through the epidermis into the dermis, whereby the first few millimetres of the five pairs of needles (representing the delivery and return electrodes) was live and the rest of the shaft was insulated, thus protecting the epidermis from initial electrothermal damage11. Cooling was still required, however, because of the long exposure times necessary to deliver the required amount of dermal electrothermal damage with this system, and the angle at which the needles were inserted into the skin. As this concept has been further refined, very sophisticated microneedling fraction RF (MFR) systems are now commercially available which allow multiple passes to deliver fractional photothermal damage at user-set decreasing depths in the dermis using insulated microneedles, but without any cooling of the epidermis required. This means that a set of layers of precise coagulative damage are interspersed with layers of undamaged skin, so wound healing is accelerated and pain is minimised. The ability to balance the power level and the exposure time with a large degree of flexibility also helps ensure that the ideal coagulative damage is delivered to optimise the wound healing process and produce good tissue tightening from deep in the dermis, working progressively upwards. As for epidermal damage, the only action of the microneedles in the epidermis is physical needling, which has been shown to help regenerate the epidermis12, and even to improve wound healing and blood circulation in skin flaps13. The evolution of the ideal MRF approach is illustrated in Figure 1.
Clinical results have been very good, irrespective of skin type or in tanned skin. Because the final conditions depend on the remodelling phase of wound healing, patients can experience continued improvement of their results for some months after the final treatment, thereby increasing the patient satisfaction with this approach, Figures 2–5 show representative examples of, respectively, MFR applied for skin tightening and lifting in an older patient, acne scars in a type IV Indian skin, and face, neck and décolleté rejuvenation in type II skin.
Light-emitting diode phototherapy
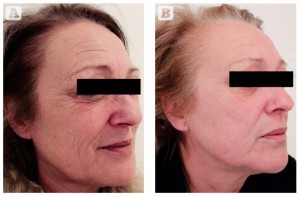
Figure 4 Photoageing of the face and neck in a 55-year-old Italian female, skin type II. (A) Baseline findings. (B) Excellent results 3 months after the second MFR treatment session, two passes per session, 2 weeks apart.
Until the late 1990s, defocused surgical lasers, such as the continuous wave Nd:YAG or CO2, or dedicated laser diode (LD)-based systems were the main light sources used in phototherapy, and many reports appeared in the literature on the efficacy, or otherwise, of this approach. Phototherapy was originally reported under a variety of good and not-so-good terms such as ‘cold laser’, ‘soft laser’, ‘low power laser’, ‘low energy laser’, ‘photobiomodulation’, ‘photobiostimulation’, ‘photobioactivation’, and ‘low level laser therapy’ or ‘LLLT’, the latter being coined by the author in 198814. Of the above terms, phototherapy, LLLT, photobiomodulation and photobioactivation have persisted, and the unification of the terminology has helped the credibility of the modality. However, some of the scientifically questionable terms still crop up, so one should be more than slightly wary of manufacturers offering ‘cold laser’ or ‘soft laser’ systems — especially when some of them are not even laser-based.
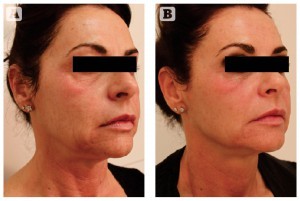
Figure 5 Photoageing of the face, neck, and décolleté in a 51-year-old Italian female, skin tye II-III. (A) Baseline findings. (B) Excellent results 3 months after the second MFR treatment session, two passes per session, 2 weeks apart.
Sadly, despite the very large body of evidence and convincing large population clinical trials found in the literature, phototherapy is still vey much a bête noire in certain medicoscientific circles, with a lot of the problem stemming from the improper and unfounded claims made by some manufacturers and, sadly, researchers without good double-blinded trials, or at the very least, a decent-sized patient population. Low populations studies have been the bane of LLLT research, e.g., ‘A study was conducted on the use of LLLT to treat cedar pollinosis. The results showed that 33.33% of the population responded to LLLT, 33.33% failed to respond, and the other patient failed to turn up for follow-up.’ What also did not help was the use of early generation light-emitting diodes (LEDs) in so-called ‘cluster probes’ for ‘cold laser therapy’ (when they were not even lasers). These LEDs were bright, cheap and cheerful but had a waveband rather than a wavelength (a ‘red’ LED emitting from 610 nm to 690 nm, for example), had unstable and low output powers and delivered a highly divergent beam.
New generation LEDs
The ambiguity surrounding LEDs started to dissipate with the development of the so-called ‘NASA LED’ by Harry Whelan and colleagues working in the NASA Space Medicine Laboratory in 199815, and LEDs became a viable phototherapeutic source because of their much higher output powers (several orders of magnitude higher), less divergence of the beam and especially their quasimonochromaticity compared with previous generation of LEDs; the last point meant that, although the new generation of LEDs were not coherent and therefore not monochromatic, a very high percentage of the emitted photons was at the rated wavelength, with the remainder at a very narrow band a few nanometres either side. It was now possible to have an LED rated at 633 nm ± 3 nm, rather than just a ‘red’ LED. Very quickly after the development of the NASA LED, Whelan et al. showed clinical efficacy for wound healing with a near-infrared wavelength of his new generation LEDs16. The first paper from another author that seriously suggested the viable application for LED phototherapy using a commercially-available LED phototherapy system for wound healing in a peer-reviewed journal came from Trelles in 200617. Due to the emergence of the LED as a viable phototherapeutic source, low level laser therapy became low-level light therapy, although the acronym remains as LLLT.
In addition to the clinical efficacy of the new generation LEDs, there are inherent benefits for LEDs over other light sources: they are very efficient, delivering a great deal of light for very little power in; they are a solid state so no flashlamps or gases are required and there are no filaments, thus minimising loss of energy to heat; they can be mounted in large planar arrays to allow hands-free irradiation of large areas of the body compared to the treatment-intensive point-by-point delivery of LDs; and finally, they are comparatively inexpensive, thereby helping to reduce the spiralling health care costs to both clinician and patient.
LED phototherapy also offers innate benefits: it can be set up and delivered by a trained nurse or therapist, relieving the load on the clinician; it is side-effect and pain free; it is well-tolerated by patients of all skin types and all ages from infants to centenarians; and most importantly, it has been proved to be safe and effective with approvals under a 510(k) existing for some systems from the US FDA. Some systems are CE marked and approvals exist under the Korean and Chinese FDA bodies; Japanese Ministry of Health, Labour and Welfare; and Australian TGA. On the other hand, there are a lot of unregulated LED-based systems available on the market, so it is very much caveat emptor, more on which later.
Optimum wavelength
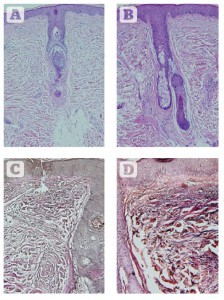
Figure 6 Histologic assessments of changes in collagen and elastin 2 weeks after eight sessions of 830 nm LED-LLLT monotherapy over 4 weeks. (A) Haematoxylin and eosin staining showing the condition of collagen at baseline. (B) 2 weeks post final treatment. A significant increase is seen in the amount and quality of collagen, with a very well-oriented Grenz layer, a thicker and more cellular epidermis, and well-organised stratum corneum. (C) Verhoeff-van Giesen stain showing the condition of the elastic fibres at baseline. (D) Findings 2 weeks after the final treatment. A significant increase in the amount and quality of elastic fibres is noted at all depths in the dermis, a thicker epidermis and well-formed stratum corneum. All at original magnification X 200.
Most wavelengths of light will elicit at least some reaction from skin cells, but a significant body of published evidence exists in the literature suggesting that the wavelength that appears to be most effective across the board for raising the action potential of all skin cells, especially those associated with wound healing, is 830 nm in the near-infrared14,18, and a selection from the most relevant articles on LED-LLLT follows.
LED phototherapy at 830 nm was shown to half the healing time post full-face ablative resurfacing, and significantly reduced the usual sequelae of erythema, oedema, and pain in a 50-patient controlled study19. In another study by the author, mast cells were shown to degranulate within 48 hours of a single 830 nm LED irradiation, a strong inflammatory response was induced and significantly large numbers of neutrophils and macrophages were recruited into the treated area compared with the unirradiated control20. The author interpreted this as a ‘quasi-wound’ in normal skin and was essential for starting off and enhancing the wound healing process, but without heat or damage. In a controlled study on the rat model axial pattern flap, enhanced vascular perfusion of the flaps after 830 nm irradiation was associated with significantly better flap survival compared with the sham irradiated models21. The same wavelength featured strongly in a very persuasive paper from Lee and colleagues, published in the Journal of Photochemistry and Photobiology22. Although the latter paper was on skin rejuvenation, good rejuvenation is underpinned by good wound healing, and Lee et al. proved this aspect with histology, immunohistochemistry and machine measurements of improved elasticity in a large population, controlled, and double blind study. The authors compared the effects of 830 nm, 633 nm, and 830 nm plus 633 nm LED phototherapy against an unirradiated control group, and all the treatment groups were also split face controlled. In a review of the photobiological basics and literature, Kim and Calderhead made a very convincing argument for the real efficacy of 830 nm LED-LLLT23. In a very recent article, 830 nm LED-LLLT proved effective in enhancing the wound healing in a variety of recalcitrant wounds including wounds complicated with bacterial infection, viral infection and prolonged inflammation24.
In order to achieve the desired clinical effect, an LED system must meet certain criteria:
- It must have appropriate wavelengths at reasonable intensities, used correctly to target and reach the desired chromophore
- It must have adequately large planar arrays, designed and easily adjusted to give even illumination of curved anatomical areas
- It should have an adequate irradiance or power density, measured in mW/cm²; the dose, in J/cm², should have been clinically assessed as the optimum. Provided the irradiance is correct, it is virtually impossible to overdose with LED-LLLT, but as treatment time is important to both clinician and patient, overdosing offers no significant benefit, although there may be a very slight improvement in the effect
- Applications of the system should be reported in a number of good clinical papers in the peer-reviewed literature
- Finally, systems should have approval from the regulatory bodies in the countries in which they are being sold: CE marking in the UK, FDA in the USA, TGA in Australia, KFDA in Korea, SFDA in China, and so on.
Provided a system meets these criteria, then using it in accordance with the manufacturer’s recommendations will produce the desired results. As in all things clinical, there will be a very few people who may not respond as well or as quickly as expected, but LED-LLLT can be expected to give some benefit to all patients, and a great deal of benefit to most.
Microneedling RF and LED-LLLT
Microneedling fractional radiofrequency (MFR) is proving extremely interesting, with all patients noticing from mild to excellent improvement. Although no clinical paper has as yet been published in the peer-reviewed literature, several are in the pipeline. In a 499 patient study that was carried out as part of the FDA approval process for one MFR system in five geographically different locations (Turkey, India, Italy Poland, and Korea), the results showed that those subjects expressing high satisfaction accounted for 80% or over of the total, and all patients noted some improvement25. Intraprocedural pain was minimal to mild, and downtime was very short with erythema peaking at 12–24 hours and naturally evolving by 48 hours. No persistent erythema or any other unwanted side-effect was seen.
In the optimum arrangement, MFR works by delivering fractional RF energy via the tips of the delivery and return needle electrodes at varying depths in the dermis, achieving discrete areas of controlled coagulation surrounded by a normal unaffected dermal matrix under a mechanically microneedled but otherwise unharmed epidermis, owing to the insulated needle shaft protecting all tissues except those at the very tip. The areas of coagulation induce the wound healing process, proceeding from inflammation, through proliferation to remodelling, the latter ensuring that good results continue to improve during the final and protracted wound healing phase process. However, even though the areas of electrothermal coagulation are well-controlled, this is still dermal damage and certainly induces the wound healing process; although very uncommon, and under less than ideal situations, this process can still be associated with some complications. Furthermore, although side-effects are usually mild and transient, there certainly are some following MFR treatment, but particularly for the more aggressive treatments which are required for severe acne scars, for example, or extensive skin laxity, the downtime for the patient can be a little longer than the usual 24–48 hours owing to the more prolonged side-effects. In addition, some patients will have more sensitive skin than others, so despite the fact that manufacturers may claim minimal pain, MFR is surely a ‘no pain-no gain’ treatment.
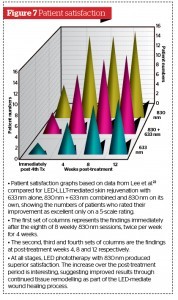
The results for MFR have been universally good to excellent. There is, however, always room for improvement, and that is where the addition of 830 nm LED-LLLT to all MFR sessions could have a very powerful synergistic effect, adding the proven healing and regenerative capability of the totally noninvasive LED energy to the already good results of the MFR. In addition, the intervention with 830 nm LED-LLLT would also help to prevent any of the slightly more extended sequelae seen following an aggressive MFR approach. Lee et al. in the paper cited above on LED-LLLT mediated skin rejuvenation clearly showed significantly improved dermal architecture at 2 weeks after the final treatment session involving both collagen and elastin deposition under a thickened and more cellular epidermis (Figure 6)22: these findings are in effect demonstrating LED-LLLT-mediated wound healing, but induced without any heat or damage. In addition, the patients’ subjective improvement in the Lee study continued to rise after the final treatment session up to the 12-week assessment, with the 830 nm-treated patients giving the best response (Figure 7), illustrating the powerful beneficial effect of the remodelling process on the final result.
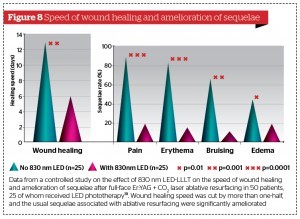
The other papers cited above on the enhanced wound healing of traumatic and iatrogenic wounds with 830 nm LED phototherapy all point to more rapid wound healing than would be expected, amelioration of post-wound sequelae, such as erythema and oedema, and pain control: this was well-illustrated in the article by Trelles et al.19 (Figure 8). In that article, the iatrogenic wounds being treated consisted of a full-face controlled second-degree burn post full-face laser resurfacing. In the case of MFR, the after-effects are really mild compared to those following full-face ablative laser treatment, but clinicians and patients will still derive great benefit from the adjunctive use of 830 nm LED phototherapy.
Conclusions
MFR produces good results in treating skin laxity, wrinkles and skin rejuvenation of the head, neck, and décolleté, as well as in other areas of the body. 830 nm LED has proven efficacy in stand-alone skin rejuvenation and enhanced wound healing with prophylaxis against hypertrophic scar formation. If these 830 nm LED-LLLT-associated benefits were to be added to good results already achieved with MFR, the author believes that an excellent synergy would be achieved delivering an outcome far superior to the effect of each modality used on its own, to the benefit of both clinicians and of course their patients.