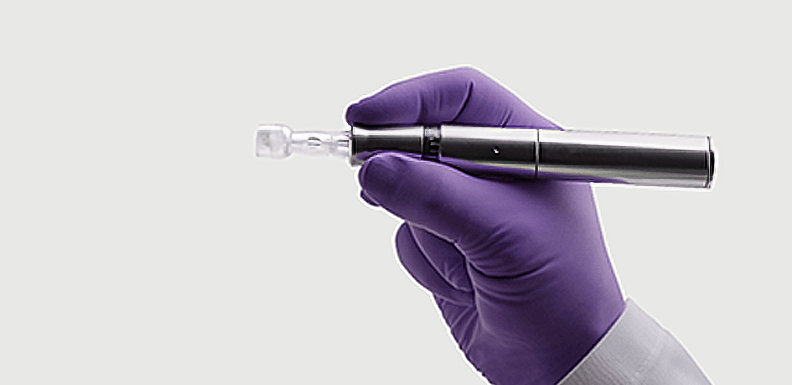
Candela Corporation, a leading global medical aesthetic device company, has entered into an exclusive collaboration agreement with MT.DERM GmbH for the commercialization of MT.DERM’s medical microneedling products. Under the terms of the agreement, Candela will commercialize the Exceed™ medical microneedling system globally, with a first launch in the United States. The agreement may be expanded with additional products in the future.
The Exceed system from MT.DERM, powered by its brand amiea med, is the world’s only dual-indicated, FDA-cleared medical microneedling device. The German-engineered device is clinically proven to dramatically reduce the appearance of facial wrinkles and facial acne scars. In parallel, it is CE registered in Europe.
Candela manufactures and distributes the Profound® system – the only real-time, temperature-controlled, long pulse radiofrequency (RF) microneedling device. With the Exceed medical microneedling system, Candela further enhances its existing microneedling portfolio and positions the company for expanded market penetration.
“We are delighted to enter into a partnership with MT.DERM, based on their superior engineering and deep understanding of needling technology” said Geoffrey Crouse, CEO of Candela. “Microneedling is an essential component of any medical aesthetics practice. The addition of the Exceed medical microneedling system strengthens Candela’s product portfolio, which continues to provide customers the most comprehensive offering of best-in-class medical aesthetic technologies.”
“MT.DERM’s value of quality and engineering excellence, partnered with Candela’s commitment to “Science, Results, Trust” along with their global customer support capability and commercial strength make this an ideal partnership for us,” added Joern Kluge, founder and CEO of MT.DERM. “Candela and MT.DERM jointly have the industry experience and reputation to make the Exceed device a leader in the mechanical microneedling global market.”
The Candela Exceed system will be available for in-office treatments by a licensed medical professional beginning in January 2020. For more information, please visit www.candelamedical.com.
Candela®
Candela Corporation is a leading global medical aesthetic device company with an extensive product portfolio and a global distribution footprint. The Company’s technology enables physicians to provide advanced solutions for a broad range of medical aesthetic applications including hair removal, wrinkle reduction, tattoo removal, women’s health treatments, facial resurfacing, traumatic and surgical scar treatments, body contouring, improving the skin’s appearance through the treatment of benign vascular and pigmented lesions, and the treatment of acne, leg veins and cellulite. Candela has a wide portfolio of trusted, leading products, including the Vbeam®, Gentle Family®, CO2RE®, CO2RE Intima®, Exceed™,
Profound®, elōs Plus®, PicoWay®, UltraShape®, VelaShape®, and IPL systems as well as other energy-based platforms. Acquired by Apax Partners in July 2017, the Company markets, services and supports its products in 86 countries. It has offices in the United States, Australia, Canada, China, France, Germany, Hong Kong, Israel, Italy, Japan, Korea, Portugal, Spain and the United Kingdom and many international distributors.
MT.DERM
MT.DERM, based in Berlin (Germany), is the global leader in microneedling, micropigmentation and tattoo devices, sold in more than 50 countries under the brands of amiea, amiea med, VYTAL and Cheyenne. Engineered and manufactured in Germany, MT.DERM focuses on innovation, high quality and precision to enable best results for doctors, cosmeticians and tattoo artists.
SOURCE Candela