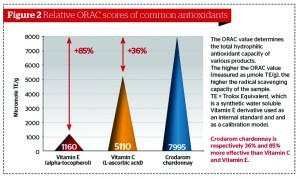
There are a number of techniques available for measuring antioxidant capacity, with the oxygen radical absorbance capacity (ORAC) score being the most frequently cited. In Figure 2 the ORAC scores of vitamin C, vitamin E, and crodarom chardonnay (chardonnay grape seed extract) are compared. The higher the ORAC score, the higher the free radical scavenging capacity of the samples that were tested. The other techniques available for measuring antioxidant capacity are:
- TEAC = trolamine equivalent absorbance capacity
- ABEL-RAC = analysis by emitted light-relative absorbance capacity
- TOSC = total oxygen radical scavenging capacity.
It is also important to understand the biological activity of antioxidants. To be effective they must not only be absorbed into the skin, but also must be present in the skin in an active form, and be available for a sufficient time to perform the intended antioxidant function. Antioxidants, by their very nature, oxidise and this presents some significant challenges in terms of delivering active (non-oxidised) antioxidants to the skin. Many topical antioxidants, such as vitamin C, are easily oxidised by oxygen and light, rendering them inactive. This leads to a search for efficient ways of stabilising topical antioxidant preparations, which is why topical vitamin C products must be stored in air-tight, light-resistant, or metallised containers9. Furthermore, vitamin C products are susceptible to changes in pH. Sheraz10 reported protonated vitamin C in a product with a pH of more than 4, which may affect pharmacological activity of the vitamin C. Many preparations have a 6-week window for use after opening, so customers should be advised to look for products with a 12-month lid symbol representing the ‘use by’ period after opening. Also, ensure the product has a ‘best before’ date, unless it has a proven lifespan of over 30 months.
Commonly used antioxidants
Commonly used antioxidants can be subdivided into fat and water soluble antioxidants. Fat soluble antioxidants are ingredients such as vitamins A and E, coenzyme Q10, idebenone, and curcumin. Water soluble antioxidants are found in hydrophobic areas of the cell and include vitamin C, glutathione, silymarin, polypodium leucomotos, grape seed extracts, and resveratrol. Alpha lipoic acid is also known to be an antioxidant, but is both water and lipid soluble.
Fat soluble forms of, for example, vitamin C (ascorbyl palmitate) are more stable than water soluble forms and they exert their effects in different areas. The water soluble forms affect the extra- and intracellular fluids, while fat soluble forms affect the lipid membrane. It is therefore ideal to have a combination of both water and fat soluble vitamin C. Other antioxidants include the phytoestrogen genistein and pinus pinaster (Pycnogenol®; Horphag Research, Geneva).
Hydroxy acids
Many topical ingredients known more for other properties are becoming recognised as powerful antioxidants. For example, Briden et al11 demonstrated that the polyhydroxy acid gluconolactone inhibits the elastin promoter gene by 50%, reducing over-production of elastin and thereby reducing solar elastosis. Clinical signs of photoageing include solar elastosis, and an antioxidant shown to reduce the appearance of solar elastosis will therefore improve cosmetic appearance.
Maltobionic acid, a third generation hydroxy acid is a powerful chelator of oxygen-promoting metals. Maltobionic acid is a bionic polyhydroxy acid consisting of a sugar acid (gluconic acid) attached to another sugar (glucose). The bionic acids are part of the alpha‑hydroxy acid (AHA) family of ingredients, and are technically polyhydroxy AHAs with multiple OH groups on the molecule12. They are called bionic acids because two sugars are bound together. One of the prominent features of the bionic/sugar acids is their water binding and hydrating potential owing to the polyhydroxy structure, and while they measure 358 Da, the molecules remain sufficiently small enough to penetrate the skin13.
Additionally, in a study by Brouda et al12, maltobionic acid was shown to prevent oxidative damage to lipids in the cell membrane and mitochondria by reducing the production of malondialdehyde, an oxidative degradation end-product, in a UV-induced lipid peroxidation test model.
Older hydroxy acids also demonstrate antioxidant activity. In a study by Higashi-Okai et al14, citric acid was shown to inhibit lipid peroxidation. Citric acid has also been shown to scavenge superoxide anion free radicals that react with nitric oxide to form peroxynitrite, which is a detrimental oxidant15. Additionally, citric acid has been shown to be synergistic with vitamin E, enhancing its antioxidant action against radiation-induced peroxidation16.