Anis Irawan Anwar, Siswanto Abdul Wahab, Widya Widita, Airin R. Nurdin, Suci Budhiani, and Arifin Seweng discuss the findings from their double-blind clinical trial
DARK SKIN COLOUR AND pigmentation abnormalities play a very important role in skin appearance and self-confidence. Asian women commonly desire to have a brighter skin colour1. Hydroquinone, the gold standard for a depigmenting agent, raised concern about its safety profile and was associated with some adverse side-effects. This has prompted research to find other therapeutic agents for hyperpigmentation2. Studies using single topical tranexamic acid have been performed as hyperpigmentation therapy (melasma) showed a decrease in the MASI score (Melasma Score Index)3.
In recent years, the approach to skin lightening has broadened. The strategy of using a single agent to inhibit tyrosinase has recently been modified by the use of combined actives with different target mechanisms. Galactomyces Ferment Filtrate (GFF) reduces the appearance of skin pigmentation by reducing melanin synthesis and oxidative stress in melanocyte cells4. Niacinamide, which in addition to being a tyrosinase inhibitor, also plays a role in blocking the transfer of melanosomes to keratinocytes5. Arbutin is a natural derivative of hydroquinone found in plants and has a mechanism of action of melanosomal tyrosinase inhibition at non-toxic concentrations6. In this study, the investigators aimed to compare the effectiveness of tranexamic acid 3% combined with other skin lightening ingredients, such as galactomyces 2%, niacinamide 4%, and alpha arbutin 2%, which are expected to exert better whitening activity versus that of 4% hydroquinone preparation and placebo.
Patients and methods
A 4 week double-blind, randomised controlled clinical trial study was conducted at the Dermatovenereology Department of Hasanuddin University, Makassar, South Sulawesi Province, Indonesia. Forty-four female Indonesian volunteers aged 25–50 years were enrolled. Samples were collected through a consecutive sampling method with the following inclusion criteria: Fitzpatrick’s classification of skin phototypes III to V and willing to participate in the study by signing the informed consent form. Exclusion criteria were pregnancy/lactation, the presence of other dermatoses on the arm, history of hypersensitivity to the substances used in this study, prior exposure to medical procedures or depigmenting agents on the face within 1 month prior to the study, and use of tranexamic acid within 3 months prior to the study. The subjects were given an explanation about the study, and those who agreed were asked to sign an informed consent form.
Results
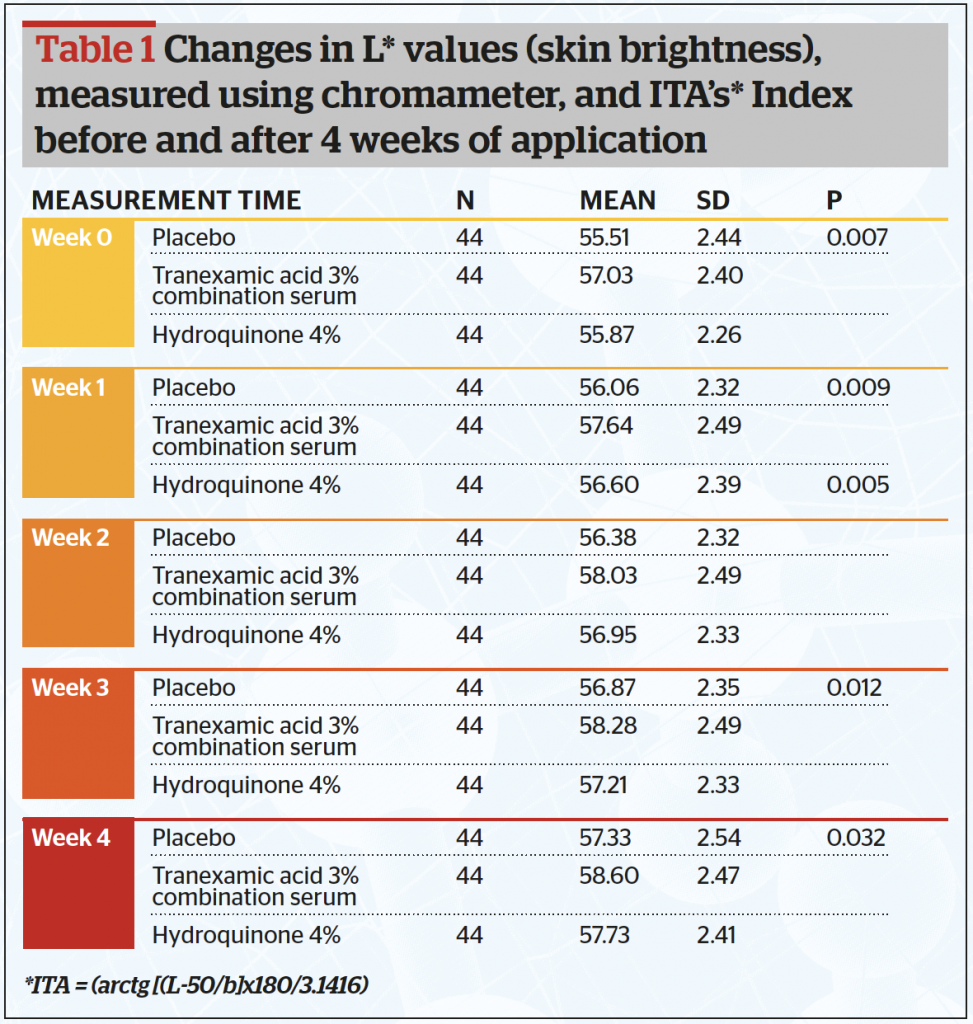
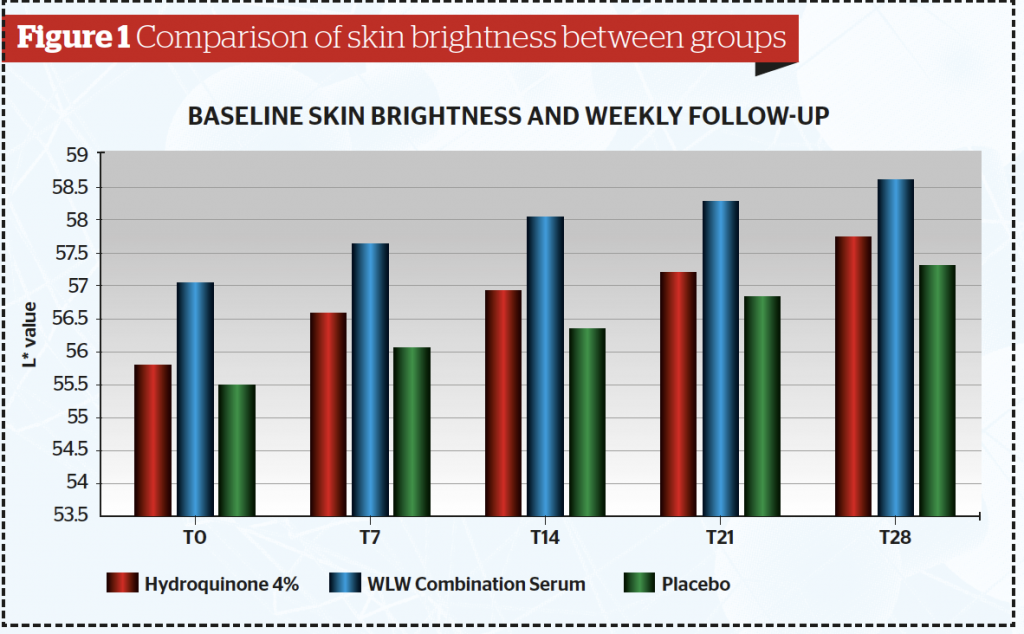
All 44 subjects completed the study. Data were analysed and tested using a one-way analysis of variance (ANOVA) test. Baseline data showed that out of the 44 samples, there were twenty-six subjects (59.1%) in the less than 30 years-old group, fourteen subjects in the 30–39 years old (31.8%) group and four patients in the >40 years (9.1%) group. Table 1 and Figure 1 show after 4 weeks of application, the mean skin brightness score was found to be highest for the combined serum (58,60) and lowest on serum B containing placebo (57,33) (p <0,05). The skin brightness for the combined tranexamic acid serum was superior to that of the hydroquinone serum.
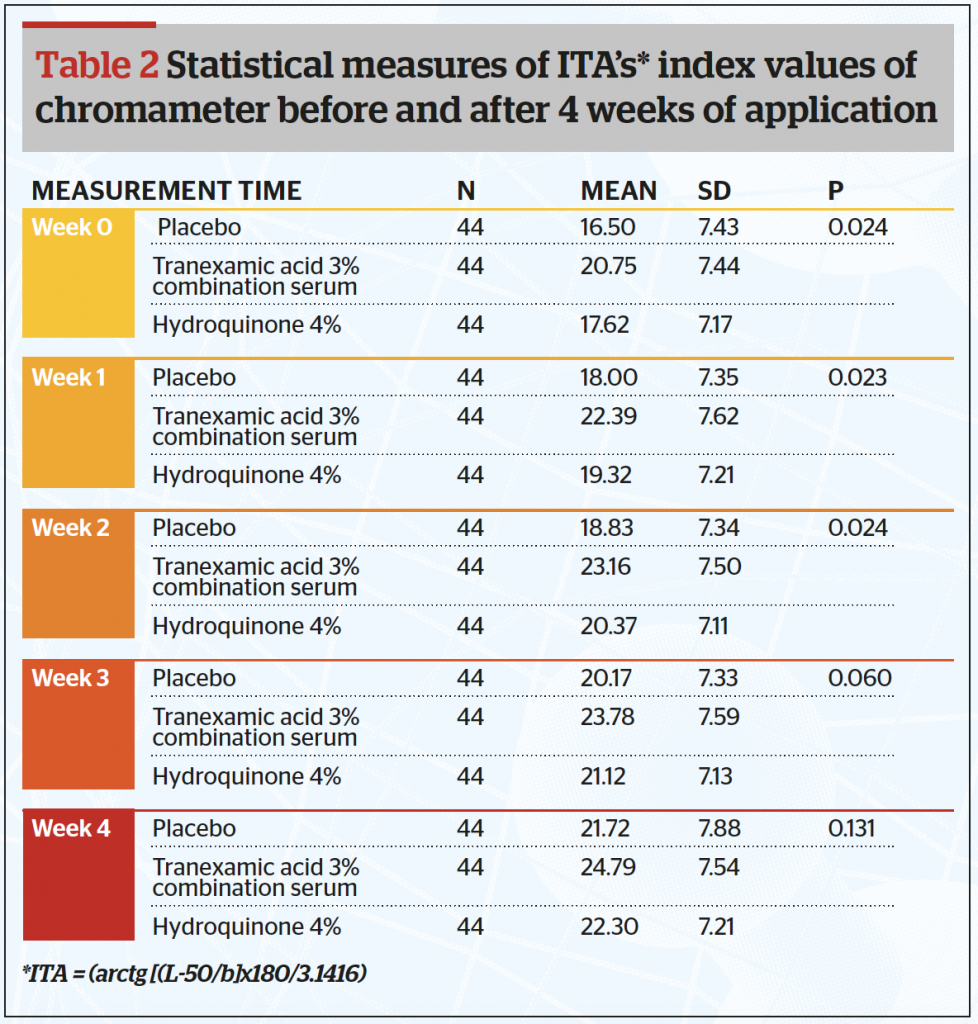
From this study, the authors calculated the Individual Topography Angle (ITA) (Table 2 and Figure 2) and found the mean ITA index at the end of the study was significantly higher in the combined tranexamic acid serum (24,79) and lowest for the placebo serum (21,72) (p<0.05). It is also clear that the ITA for the combined serum is higher than that of the hydroquinone serum.
No specific adverse events were observed during the study period.
Discussion
The results of this study were consistent with that of Kim et al. 2016, where they also examined the efficacy of topical tranexamic acid as a depigmentation agent. Several studies have been performed to find possible mechanisms of action of tranexamic acid as a melasma treatment. Ultraviolet exposure increases plasminogen activator production by epidermal keratinocytes in situ, resulting in the secretion of melanogenic factors such as prostaglandins. Tranexamic acid (trans-4-aminomethyl cyclohexanecarboxylic acid) exerts its effects on melasma by reversibly blocking lysine binding sites on plasminogen7–9. Tranexamic acid is a natural plasmin inhibitor that blocks the conversion of plasminogen to plasmin. The drug accomplishes this through the inhibition of plasminogen activators by creating a reversible complex with plasminogen10. Plasmin is a protease that enhances the intracellular release of arachidonic acid (AA) and alpha-melanocyte-stimulating hormone (α-MSH). AA and α-MSH have the property of stimulating melanogenesis by melanocytes. Tranexamic acid, being a plasmin inhibitor, depletes the keratinocyte pool of AA involved in UV-induced melanogenesis8,11.
This study demonstrated that at various time 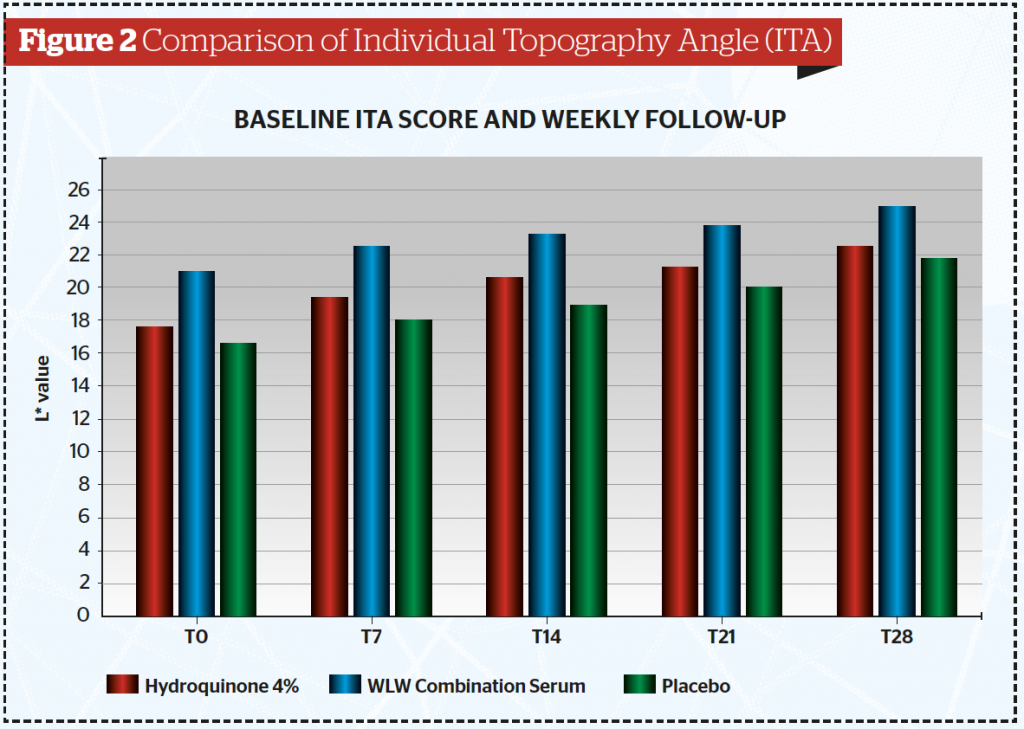
The result of this study has an important implication that by having the right combination of several whitening agents with different whitening mechanisms, it is possible to have an alternative whitening agent as powerful as hydroquinone, which has been the gold standard for many years. The combined tranexamic acid serum can be a good alternative to hydroquinone since there were no side-effects observed during the study. However, more studies will have to be performed for more extended periods of time and for various skin pigmentation disorders.
Conclusion
This study showed serum containing a combination of tranexamic acid 3%, galactomyces ferment filtrate 2%, niacinamide 4% and alpha arbutin 2% can be considered as a safe and effective alternative to increase skin brightness with no significant side-effect.
Declaration of funding: No funding or sponsorship was received for this study or publication of this article.
Declaration of financial/other relationships: All authors have no affiliation with or financial involvement in any organisation or entity with a direct financial interest in the subject matter or material discussed in the manuscript.
Declaration of interest: The authors report no conflicts of interest
We would like to thank PT. LipWih SynergyLab in the making of all serums that were used in this study. All the named authors take responsibility for the integrity of the work as a whole and have given final approval for the version to be published
Compliance with Ethics Guidelines: This study was approved by the Ethics Committee of the Hasanuddin University, Medical Department
Figures 1–2 © Dr Anwar Tables 1–2 © Dr Anwar
References:
- Baumann L AI. Depigmenting Agent. In: Baumann L, Saghari S, Weisberg E (Eds). Cosmetic Dermatology. 2 ed: McGraw Hill; 2009
- Kindred C, Okereke U, Callender V. Skin-Lightening Agents: An Overview of Prescription, Office-Dispensed, and Over-the-counter Products2013. 18-23 p
- Poojary S MK. Tranexamic Acid in Melasma: A Review. J Dermatol Dis. 2015;2(228)
- Woolridge J, Koshoffer, A., Kadekaro, A. L., Wickett, R. R., Boissy, R. E. & Hakozaki, T. Galactomyces ferment filtrate reduces melanin synthesis and oxidative stress in normal human melanocytes. J Am Acad Dermatol. 2014;70(5):AB127
- Smit N, Vicanova J, Pavel S. The Hunt for Natural Skin Whitening Agents. Int J Mol Sci. 2009;10(12):5326-49
- Sugimoto K, Nishimura T, Nomura K, Sugimoto K, Kuriki T. Inhibitory effects of alpha-arbutin on melanin synthesis in cultured human melanoma cells and a three-dimensional human skin model. Biol Pharm Bull. 2004;27(4):510-4
- Kim SJ, Park JY, Shibata T, Fujiwara R, Kang HY. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41(5):480-5
- Tse TW, Hui E. Tranexamic acid: an important adjuvant in the treatment of melasma. J Cosmet Dermatol. 2013;12(1):57-66.
- Maeda K, Naganuma M. Topical trans-4-aminomethylcyclohexanecarboxylic acid prevents ultraviolet radiation-induced pigmentation. Journal of Photochemistry and Photobiology B: Biology. 1998;47(2):136-41
- Kim MS, Bang SH, Kim JH, Shin HJ, Choi JH, Chang SE. Tranexamic Acid Diminishes Laser-Induced Melanogenesis. Ann Dermatol. 2015;27(3):250-6
- Calapai G, Gangemi S, Mannucci C, Minciullo PL, Casciaro M, Calapai F, et al. Systematic review of tranexamic acid adverse reactions. Journal of Pharmacovigilance. 2015;2015
- George A. Tranexamic acid: An emerging depigmenting agent. Pigment Int. 2016;3(2):66-71
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19(8):753-7
- Hakozaki T, Minwalla L, Zhuang J, Chhoa M, Matsubara A, Miyamoto K, et al. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br J Dermatol. 2002;147(1):20-31.