Hernán Pinto and Graciela Melamed share the results of their study examining the change to PRP growth factor content when exposed to thermal conditioning for ten minutes

i2e3 Biomedical Research Institute, Barcelona, Spain;
Graciela Melamed, MD
GM Clinic, Buenos Aires, Argentina
email [email protected]
There is currently great interest in the procedures and handling that some patient blood preparations may be subjected to in order to achieve the desired result. The typical example is platelet-rich plasma and/or plasma rich in growth factors1. This type of autologous material has many variants, including several duly registered products that, in this study, will be grouped under the generic name of PRP2.
In the last two decades, PRP application has been made accessible to all; however, since it became a product mentioned in almost every aesthetic medicine consultation, its development has not been without complications3. PRP application is currently very well regulated in most countries and the technique has evolved4. Nevertheless, this evolution has been inconsistent5,6. On the one hand, there has been a marked advance regarding fungible materials. On the other, the technique has undergone moderate advances, while the end product has not really improved in years5,7,8.
As for the material used, no doubt the emergence of kits specially made to obtain PRP has entailed a significant improvement, making the procedure easier and at the same time promoted the proper implementation of a ‘closed’ technique. Later, devices to obtain and separate PRP into phases also meant a wonderful advance, especially at a later time, when the lowering of its market price made it affordable for all. However, the evolution of PRP as a therapeutic product became stagnant9.
Reports on the concentration of growth factors and cytokines that can be obtained with PRP have not changed much in recent years10. In fact, the conditioning entailed by PRP activation has not significantly varied, whether in concentration or the activators chosen, which are still mostly calcium chloride and gluconate. However, new conditioning protocols are changing the game. In this context, conditioning stands for the controlled exposure of the autologous material to a certain physical and/or chemical stimulus, relying on the fact that the exposure itself will determine changes in the material that will ultimately lead to an enhancement of its clinical capabilities and curative potential11.
The benefits of tissue photoactivation are increasingly being reported with more frequency12,13. There is currently an array of evidence on the positive effects of tissue conditioning. Furthermore, it would seem that these beneficial effects could be transversal since reports come from multiple medical specialties. However, most of the evidence is from studies in which this conditioning was physical (not chemical) and, in general, used light: that is, photoconditioning or, in other words, photoactivation or photomodulation12. But light energy requires a chromophore or a biological structure able to absorb it. This obstacle, however, is not present when thermal energy is used, which is also useful for conditioning procedures, and is known as thermal conditioning.
It is a known fact that cold affects organisms in multiple ways and is able to alter all kinds of processes, including the concentration of growth factors found in PRP, based on variables, such as time or temperature of exposure14. On the other hand, the laws of thermodynamics perfectly describe how thermal exchange processes between objects are regulated, which makes thermal conditioning highly affordable and attractive at the same time.
There are both general and specific precedents of thermal conditioning. First, all studies describing some of the changes occurring in liquid tissue preparations subject to certain temperatures must be taken into account15. Second, there are precedents of thermal conditioning studies in liquid autologous tissues, like blood serum. Lastly, we currently have specific evidence about the positive effect that cold has on PRP15.
The main objective of this study has been to evaluate the effects of a thermal conditioning protocol in a liquid autologous tissue (PRP) using an innovative, pioneering set-up.
Materials and method
Platelet-rich plasma was obtained from 16 patients with a mean (SD) age of 44 (10) years, consecutively recruited throughout the months of October and November 2019. Inclusion criteria were healthy patients, no allergies or atopy conditions, and no concomitant medications.
To prepare 8 ml of platelet-rich plasma, 40 ml of blood was drawn from each patient with the traditional, closed, calcium-chloride-activated technique, and without using any commercial kits: the extraction was performed with a Vacutainer® system, Terumo® 4-ml tubes with sodium citrate 3.8%, 0.05 ml of CaCl-10% per ml of PRP, 1500 RPM, 7 min, 600 Gs centrifugation cycle, and the lower third of the plasma fraction from each tube was collected in a 10 ml syringe.
Two samples of PRP—before and after thermal conditioning—from each patient were analyzed. The content of plasma cytokines in the samples was measured using the Proteome Profiler™ Human Cytokine Array Kit (R&D Systems, Minneapolis, USA). The system is semi-quantitative and allows the user to measure variations (increases or decreases) in the concentration of reactants through image density observed in the X-rays.
Each sample was lodged in an MCT®K receptacle (MCT, Sant Cugat, Spain), where they were subjected to a thermal activation protocol with the MCT® System activator (MCT, Sant Cugat, Spain) at 4 °C for 10 minutes. The temperature was controlled by the algorithm included in the activator itself and compared in real-time with a GrandBeing GM1312 liquid probe simultaneously hooked up.
Ethical considerations
The study was conducted in accordance with the principles set forth in the current revised version of the Declaration of Helsinki, with Good Clinical Practice (GCP) and all participants signed informed consent for the transfer of their samples for the study. The collection and management of data were carried out in accordance with Organic Law 15/1999 on Protection of Personal Data and Regulation (EU) 2016/679 of the European Parliament and of the Council of April 27, 2016, regarding the protection of natural persons concerning the processing of personal data and the free movement of these data.
Results
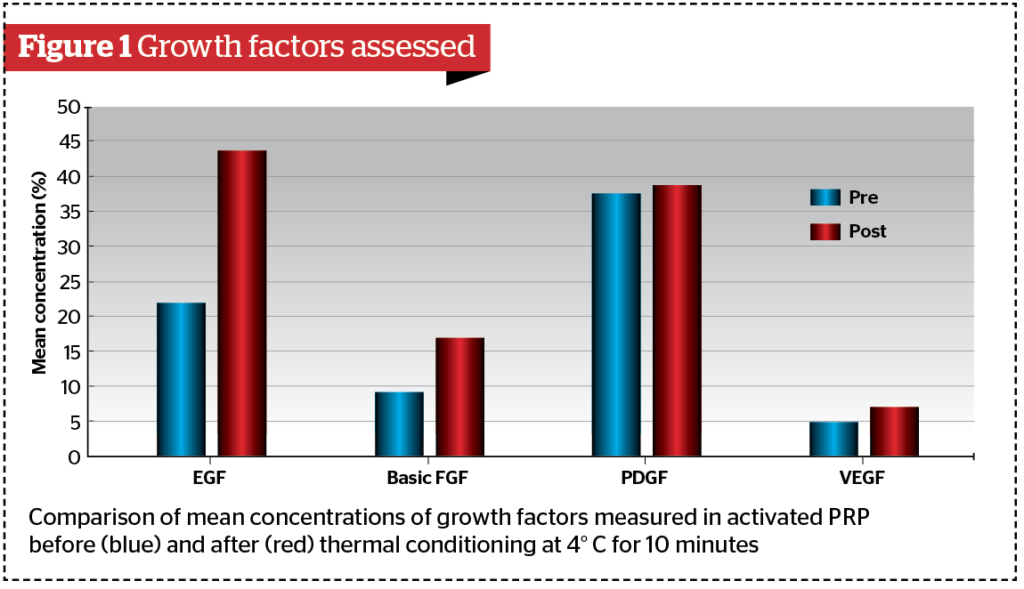
Concentrations before (pre-TC) and after (post-TC) thermal conditioning are described as mean and standard deviations (Figure 1). Epidermal growth factor (EGF): pre-TC 21.98 (3.41), post-TC 43.69 (4.05); p-value <0.001. Basic fibroblast growth factor (FGF): pre-TC 9.188 (1.94), post-TC 16.981 (2.57); p-value < 0.001. Platelet-derived growth factor (PDGF): pre-TC 37.44 (2.80), post-TC 38.65 (2.97). p-value = 0.24. Vascular endothelial growth factor (VEGF): pre-TC 4.88 (0.89) and post-TC 6.99 (0.79); p-value<0.001.
Discussion
Results show that the differences between pre-TC and post-TC of the epidermal growth factor (EGF), the basic fibroblast growth factor (basic FGF) and the vascular endothelial growth factor (VEGF) are statistically significant (p-value<0.001), while the difference between pre-TC and post-TC of the platelet-derived growth factor (PDGF) is not (p-value=0.24). In our study, the increase observed in post-TC EGF concentration was 98.8%, the increase observed in post-TC basic FGF was 84.82%, and that observed for post-TC VEGF was 43.24%.
These findings are consistent and in line with the general evidence reported in other studies. However, the difference is not as significant as those reported by other groups — up to twice as much as what we have observed in our study. On the one hand, this accounts for the unlimited possibilities of any conditioning of autologous materials, and, in this particular case, thermal conditioning. On the other hand, this disparity in the reports reveals the fact that the reason why it occurs is unknown, and that the cause may be spread out in multiple factors. One of the most likely reasons is the time of thermal conditioning. In this study, samples were conditioned for 10 minutes, while other studies that have reported a higher increase in post-TC conditioned the samples at 4 °C, for 30 minutes or more.
Future studies must shed light on matters as important as this one and set the foundation of thermal conditioning protocols. More studies assessing the perfect temperature and total conditioning time to which tissues are subjected are necessary, as well as protocols examining the concentration variation of a larger number of tissue proteins, which should not be limited only to growth factors. Finally, said assessment must be performed with a larger sample and methods of quantitative analysis.
Lastly, and however, it is worth noting the huge clinical potential of thermal conditioning. Data obtained in this study allow us to establish the suitability of this system as an effective tool to take clinical advantage of thermal conditioning in the future.
Future clinical studies must assess the clinical benefit of this treatment and transform this innovative, reliable tool in effective therapeutic protocols that are able to provide benefits for patients in endless clinical contexts. From here on, a huge range of possibilities opens up, where each specialist can suggest, with guarantees, the use of thermal conditioning in a safe and reliable way for different pathologies and with different goals.
Declaration of interest None
Acknowledgements The author would like to thank the medical writer team of i2e3 Biomedical Research Institute, and especially Elena Sánchez-Vizcaíno Mengual and Paloma Goñi Oliver
Figure 1 © Dr Pinto
Images © Shutterstock.com
REFERENCES
- Middleton KK, Barro V, Muller B, Terada S, Fu FH. Evaluation of the effects of platelet-rich plasma (PRP) therapy involved in the healing of sports-related soft tissue injuries. Vol. 32, The Iowa orthopaedic journal. 2012. p. 150–63
- Arora S, Kotwal U, Dogra M, Doda V. Growth Factor Variation in Two Types of Autologous Platelet Biomaterials: PRP Versus PRF. Vol. 33, Indian Journal of Hematology and Blood Transfusion. Springer India; 2017. p. 288–92
- Alser OH, Goutos I. The evidence behind the use of platelet-rich plasma (PRP) in scar management: a literature review. Scars, Burn Heal. 2018 Jan;4:205951311880877
- Jones IA, Togashi RC, Thomas Vangsness C. The Economics and Regulation of PRP in the Evolving Field of Orthopedic Biologics. Vol. 11, Current Reviews in Musculoskeletal Medicine. Humana Press Inc.; 2018. p. 558–65
- Maria-Angeliki G, Alexandros-Efstratios K, Dimitris R, Konstantinos K. Platelet-rich plasma as a potential treatment for noncicatricial alopecias. Vol. 7, International Journal of Trichology. Medknow Publications; 2015. p. 54–63
- Goh C. The need for evidence-based aesthetic dermatology practice. J Cutan Aesthet Surg. 2009;2(2):65.
- Du L, Miao Y, Li X, Shi P, Hu Z. A novel and convenient method for the preparation and activation of PRP without any additives: Temperature controlled PRP. Biomed Res Int. 2018;2018.
- Xie X, Zhang C, Tuan RS. Biology of platelet-rich plasma and its clinical application in cartilage repair. Vol. 16, Arthritis Research and Therapy. 2014.
- Jain A, Bedi RK, Mittal K. Platelet-rich plasma therapy: A novel application in regenerative medicine. Vol. 9, Asian Journal of Transfusion Science. Medknow Publications; 2015. p. 113–4.
- Pochini A de C, Antonioli E, Bucci DZ, Sardinha LR, Andreoli CV, Ferretti M, et al. Analysis of cytokine profile and growth factors in platelet-rich plasma obtained by open systems and commercial columns. Einstein (Sao Paulo). 2016 Jul 1;14(3):391–7.
- Demidova-Rice TN, Wolf L, Deckenback J, Hamblin MR, Herman IM. Human platelet-rich plasma- and extracellular matrix-derived peptides promote impaired cutaneous wound healing in vivo. PLoS One. 2012 Feb 23;7(2).
- Priglinger E, Maier J, Chaudary S, Lindner C, Wurzer C, Rieger S, et al. Photobiomodulation of freshly isolated human adipose tissue-derived stromal vascular fraction cells by pulsed light-emitting diodes for direct clinical application. J Tissue Eng Regen Med. 2018;12(6):1352–62.
- Tani A, Chellini F, Giannelli M, Nosi D, Zecchi-Orlandini S, Sassoli C. Red (635 nm), near-infrared (808 nm) and violet-blue (405 nm) photobiomodulation potentiality on human osteoblasts and mesenchymal stromal cells: A morphological and molecular in vitro study. Int J Mol Sci. 2018 Jul 3;19(7).
- Wen YH, Lin WY, Lin CJ, Sun YC, Chang PY, Wang HY, et al. Sustained or higher levels of growth factors in platelet-rich plasma during 7-day storage. Clin Chim Acta. 2018 Aug 1;483:89–93.
- Etulain J, Mena HA, Meiss RP, Frechtel G, Gutt S, Negrotto S, et al. An optimised protocol for platelet-rich plasma preparation to improve its angiogenic and regenerative properties. Sci Rep. 2018;8(1):1–15