Treatments for melasma
Melasma may cause a significant negative impact on the patient’s psychology and their quality of life. Key principles of therapy include the inhibition of the activity of melanocytes, the inhibition of the synthesis of melanin, the removal of melanin, and the disruption of melanin granules (Table 1). Management of melasma includes the avoidance of precipitating factors, and the use of topical bleaching agents including phenolic compounds, non-phenolic compounds, and combination formulas. Also, chemical peels, lasers and light sources may be used. Treatment should be individualised and tailored to each patient’s needs. An important challenge with melasma treatment is the fact that it commonly relapses after treatment and necessitates maintenance therapy.
Avoidance of precipitating factors
UV and visible light can initiate or aggravate melasma. The regular use of broad spectrum (UVA, UVB) sunscreens is necessary both for preventing melasma and for enhancing the effectiveness of topical therapies17. Women with melasma receiving oral contraceptives (OC) should discontinue them if possible and choose another method of contraception. However, melasma may persist despite OC discontinuation18.
Bleaching agents
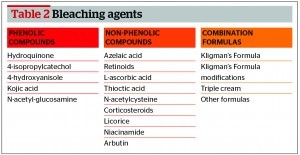
Bleaching agents include phenolic compounds, non‑phenolic compounds and combination formulas (Table 2).
Hydroquinone
Hydroquinone (HQ) has been used for over 50 years for the treatment of melasma. It is considered to be the most efficacious bleaching agent. It inhibitis tyrosinase activity, and therefore inhibits the conversion of tyrosine to melanin and increases the degradation of melanosomes. It is active only in epidermal melanoyctes that have tyrosinase activity, while it is inactive for dermal melanophages10. The efficacy of HQ is related to:
- Concentration
- Vehicle
- Chemical stability.
The use of HQ 3–5% is recommended for melasma, while lower concentrations of 2% are used as maintenance therapy10.
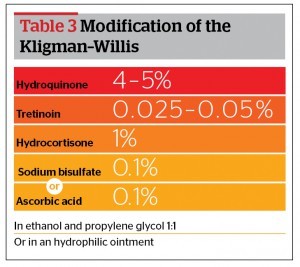
An HQ formula is made either in a cream base, a gel (AHA 10%), or in ethanol and propylene glycol (1:1) with ascorbic acid 0.1% added as an antioxidant agent20.
Side-effects from HQ are dose-related and they are associated with the concentration used and the duration of application. They include irritant or allergic contact dermatitis, nail discolouration, guttate hypomelanosis, and exogenous ochronosis.
Exogenous ochronosis is defined as the permanent grey–brown or blue–black hyperpigmentation, with pinpoint, dark brown (caviar-like) papules, that usually result from prolonged application of high concentration HQ, although it has been reported with application of HQ 2%21. The mechanism of ochronosis involves the inhibition of the enzyme homogentisic oxidase by HQ and the accumulation of homogentisic acid that is polymerised to form ochre pigment in the papillary dermis.
HQ is contraindicated during pregnancy and lactation. In the European Union, HQ has been banned from use as a cosmetic ingredient since 2001 for concerns such as ochronosis and occupational vitiligo. However, it is still available as prescription medication22.
Non-phenolic compounds
Kojic acid
Kojic acid is the second most effective non-prescription bleaching agent. It is a fungal metabolic product (Aspergillus, Penicillium), which inhibits tyrosinase acitivity. It may cause sensitisation and allergic contact dermatitis. Also, it has been found to be mutagenic in cell culture studies23. Sun exposure should be avoided during use of kojic acid. It may be used alone at a concentration of 1–4% twice daily or as combination treatment together with glycolic acid 5%24–26.
Azelaic acid
Azelaic acid is a dicarboxyl acid derived from Pityrosporum ovale (Malassezia ovalis). It acts by inhibiting tyrosinase and selectively affects hyperactive melanocytes without acting on normal melanocytes. Also, it possesses an anti‑inflammatory and cytotoxic action on melanocytes. It is an off-label treatment for melasma. Twice daily, long-term application is needed, often more than 3 months. It is very well tolerated24,27.
Azelaic acid (15% gel, 20% cream) has been studied compared to HQ for the treatment of melasma. A double‑blind, randomised study of azelaic acid 20% compared to HQ 2% in 155 patients for 6 months reported good to excellent overall results in 73% of the azelaic acid patients, compared with 19% of the hydroquinone patients28. Another double-blind study of 329 women for 6 months showed similar efficacy for azelaic acid 20% with HQ 4% (71% median lesion size reduction of melasma compared to 78%, respectively)29. Also, azelaic acid cream 20% has been used as a combination therapy with glycolic acid 15% or 20%, with similar efficacy with HQ 4% alone30.