Aleksandar Godic takes an in depth look at the use of adipose-derived stem cells to address facial ageing
AGEING IS THE RESULT OF TWO overlapping processes, intrinsic and extrinsic ageing. Intrinsic structural changes occur as a consequence of physiological ageing and are genetically determined1–3. The rate of ageing varies significantly among individuals and even among different anatomic sites in a single individual. Many theories have tried to explain the ageing process, but the most plausible of these focus on DNA damage and repair processes, which induce genome-wide epigenetic changes leading to cell senescence, loss of proper cell function, and genomic aberrations4. The cell signals from DNA damage lead to three possible responses: transient cell cycle arrest (repair), stable cell cycle arrest (senescence) or cell death (apoptosis). Intrinsic (genetically determined or chronologic) and extrinsic (UV and toxic exposure–mediated) ageing processes overlap and are strongly related to increased generation of free radicals in cells, tissues, and organs. The underlying mechanism of both processes is increased oxidative stress, which is probably the single most harmful contributor to ageing, leading to the loss of cells and extracellular matrix5.
Manifestations of facial ageing
The clinical manifestations of intrinsic ageing reflect the balance between the severity of tissue damage and their regenerative abilities. All proliferating and terminally differentiated cells are susceptible to harmful events leading to intrinsic ageing6. The signs of facial ageing generally start early, with most tissue and cells gradually becoming aged and less efficient. The facial skin becomes thin and transparent. Multiple hyperpigmentations, hypo-pigmentations, and telangiectasias appear due to ageing and exposure to harmful events, especially sun damage.
The facial bones, cartilage, and muscles become atrophic. There is a loss of underlying subcutaneous fat from the upper face and redistribution and accumulation to the lower-third of the face. These changes collectively lead to a change of the facial contour, which includes hollowed cheeks, sunken eye sockets, flattened forehead and temples, ptosis of the eyebrows and the eyelids, droop of the nasal tip, retraction of the chin, atrophy of the ear lobes and the lips, thickened neck, and angulated shape of the lower face. The facial skin becomes atrophic and sags due to a loss of underlying support, decreased elasticity, and the influence of gravity. Consequently, dynamic and static skin lines, wrinkles, and furrows develop with time7. In addition, the reflection of light and shadows on convexities and concavities of the face create the illusion of the tired face.
Intrinsic (genetically determined or chronologic) and extrinsic (UV and toxic exposure–mediated) ageing processes overlap and are strongly related to increased generation of free radicals in cells, tissues, and organs.
Greater understanding of volume loss as a critical component of facial ageing and non-surgical volume replacement is the most significant recent development in the field of facial rejuvenation. Autologous adipose-derived stem cells have the potential to address facial ageing as described above.
Stem Cells
Stem cells are capable of extensive self-renewal and expansion and have the potential to differentiate into any type of somatic tissue8. They can be used in regenerative medicine, reconstructive surgery, and tissue bioengineering and can be derived from a number of tissues. Embryonic stem cells (ESC) are derived from human embryos from couples that undergo in vitro fertilisation, raising concerns about the ethics and the possibility of rejection. In addition, there is a concern of rejection reactions in non-related donors9. Induced pluripotent stem cells (iPSC) are derived from modified differentiated adult somatic cells and have similar properties as ESC. They are more acceptable because they are not derived from human embryos but involve major genetic modifications in in vitro conditions before they can be used for research and in clinical practice10,11.
Adipose-derived stem cells (ASCs)
Autologous adult stem cells are immunocompatible, and no ethical concerns exist related to their use. Multipotent mesenchymal stem cells (MSC), which have similar characteristics to bone marrow-derived MSC, are nonhaematopoietic cells from the mesoderm and are present in various postnatal organs and connective tissues: trabecular bone12, periosteum13, synovial membrane14, skeletal muscle15, skin16, pericytes17, peripheral blood18, deciduous teeth19, periodontal ligament20, and the umbilical cord21,22. Adult stem cells derived from those tissues would require ex vivo expansion or manipulation before they could be used clinically because quantity in such tissues is low.
Multipotent stem cells within adipose tissue, termed adipose-derived stem cells (ASCs)23, are one of the most promising stem cell populations identified thus far because human adipose tissue can be easily harvested with minimal liposuction and does not cause patient discomfort. Autologous ASCs have been shown to be safe and effective in preclinical and clinical studies24,25. To date, a number of scientific papers on ASCs biology and their use in regenerative medicine have been published, and their efficacy has been determined in several clinical trials.
Localisation and cellular characteristics of ASCs
Adipose tissue is composed mainly of adipocytes (fat cells), which are clustered into fat lobules26. They consist of mature adipocytes (more than 90% of the tissue volume), and a stromal vascular fraction (SVF), composed of preadipocytes, fibroblasts, vascular smooth muscle cells, endothelial cells, resident monocytes/macrophages, lymphocytes, and ASCs27,28. Characteristics of ASCs differ according to the location of the harvested adipose tissue. Most resistant to apoptosis are ASCs harvested from superficial abdominal regions, followed by those harvested from the medial thigh, trochanteric, and superficial, deep abdominal depots29. The density of stem cells varies among different locations and types. They are most abundant in the subcutaneous compartment of white adipose tissue compared to visceral fat30.
To date, a number of scientific papers on adipose-derived stem cell biology and their use in regenerative medicine have been published, and their efficacy has been determined in several clinical trials.
ASCs has been found within the brown adipose tissue, which possess skeletal myogenic differentiation potential31. Freshly isolated stromal vascular fraction (SVF) is a heterogeneous cell population that includes ASCs, endothelial cells, vascular smooth muscle cells, pericytes, and hematopoietic cells in uncultured conditions32. Freshly isolated SVF and those after few divisions express higher levels of CD117 (c-kit), human leukocyte antigen DR (HLA-DR), and stem cell-associated markers (e.g. CD34), and lower levels of stromal cell markers22,32–48. As they proliferate, they lose CD34 surface antigen44. Adipose-derived stem cells, which express CD34+, have a greater proliferative capacity, while those, which do not express CD34, are more plastic33,49. They share many cell surface markers with pericytes and bone marrow MSC33. Adipose-derived stem cells are most likely located within the perivascular region because they express pericytes surface antigens50,51. Adipose-derived stem cells have the capability to divide, self-renew, and proliferate due to their telomerase activity, which is diminished as they age52. They do not exhibit immunosuppressive properties because they do not express HLA-DR antigens on their surface53.
The role of ASCs in regenerative medicine
Previous studies have suggested that ASCs exhibit their beneficial effects (angiogenesis, anti-inflammation, and anti-apoptosis) through the secretion of cytokines and growth factors rather than by their differentiation into various cell types54,55. The ASCs cytokines and growth factors have the potential to be used in cell-based treatments in regenerative medicine. A number of papers have described the composition of the secretory factors of pre-adipocyte, ASCs, and adipose tissue56,57. The cultured ASCs (after a few divisions), secrete adiponectin, angiotensin, basic fibroblast growth factor (bFGF), cathepsin D, CXCL12, granulocyte-macrophage colony-stimulating factor (GCSF), hepatocyte growth factor (HGF), insulin-like growth factor (IGF)-1, interleukins 6–8 and 11, pentraxin, pregnancy zone protein, retinol-binding protein, transforming growth factor-β (TGFβ), tumor necrosis factor-α (TNFα), and vascular endothelial growth factor (VEGF)56–58.
Proliferation capacity of ASC
Previous reports have shown that ASCs double in number from 40 to 120 hours, depending upon age of donor, type of adipose tissue (white versus brown), its location (subcutaneous versus visceral), the harvesting procedure, and culture conditions23,43,59,60. The proliferation capacity of ASCs is highest in young individuals and diminishes as we age. Adipose-derived stem cells also gradually lose proliferative capacity with passaging in in vitro conditions60. Senescence of ASCs is similar to bone marrow-derived MSC59. Adipose-derived stem cells remain stable as they proliferate in culture and do not change their diploid karyotype61. Their proliferation can be stimulated by various growth factors, among which fibroblast growth factor 2 (FGF-2) is most important and required for their self-renewal62–64. The proliferation of ASCs can also be stimulated by platelet-derived growth factor (PDGF) and oncostatin M65,66. Adipose-derived stem cell proliferation can also be stimulated by growth factors supplemented by thrombin-activated platelet-rich plasma67, human platelet lysate68, and human thrombin69.
Differentiation potential of ASCs
Adipose-derived stem cells have the capacity to differentiate into mesoderm, ectoderm, and endoderm lineage cells, although they are of mesodermal origin70,71. They can differentiate into adipogenic72–74, osteogenic75, chondrogenic75–77, myogenic78, cardiomyogenic79,80, angiogenic81, tenogenic82, and periodontogenic lineages83. There have been few studies published regarding their ectodermal differentiation potential. One group has described epithelial differentiation of cultured ASCs, which express the epithelial markers such as cytokeratins 8 and 18, and E cadherin84,85. In another study, the differentiation of ASCs were associated with retinal pigmented epithelium ectodermal origin86. ASCs under cultured conditions can differentiate into neuronal or neuronal precursor cells87.
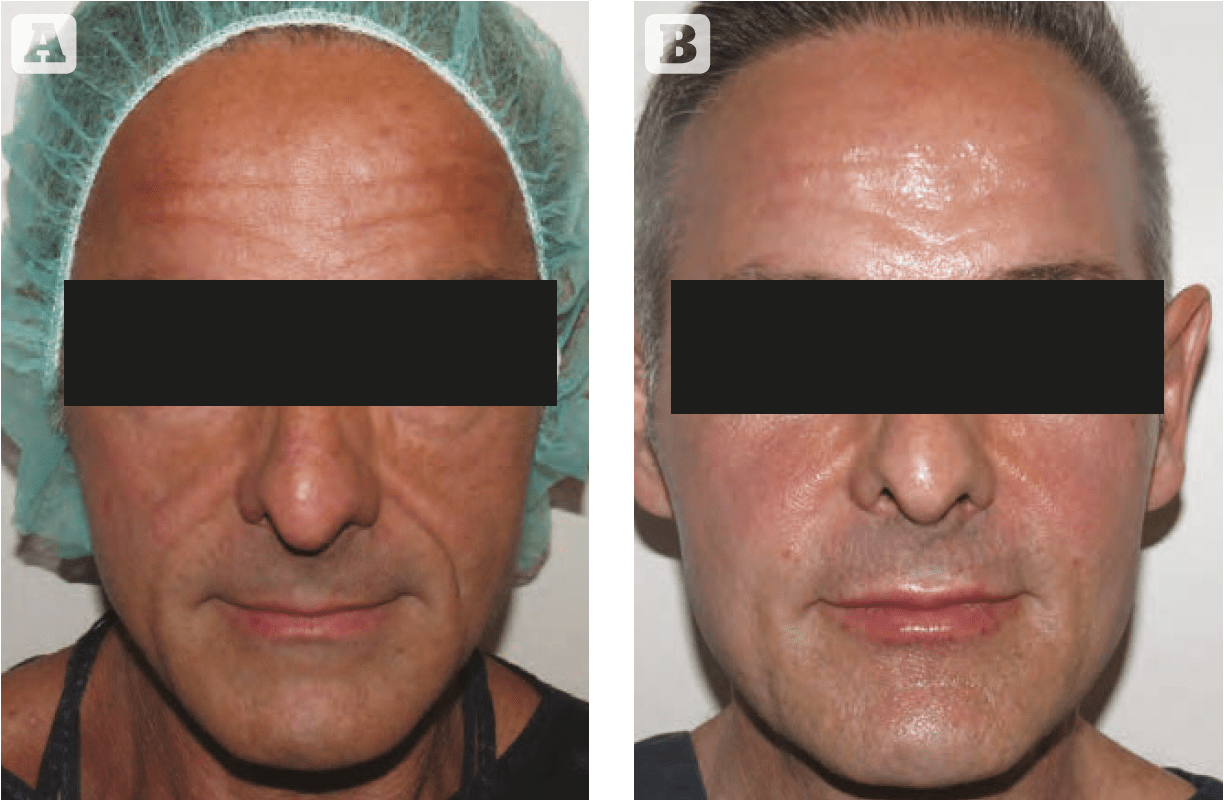
Figure 1 52 year old male (A) before and (B) immediately after the injection of autologous adipose-derived stem cells (performed by Dr Dimitra Dasiou)
Intravenous administration of ASCs in animal models on brain ischaemia or haemorrhage demonstrated functional and histological improvement88,89. In addition, recent studies have revealed the beneficial effect of intravenously administered ASCs in animals with a spinal cord injury since they migrated and partially differentiated into neurons and oligodendrocytes, and restored locomotor function90. Last but not least, it has been demonstrated that ASCs can differentiate into endoderm lineage cells. It has been published that ASCs have the potential to differentiate into hepatocytes91,92, which raises the possibility for them to be used to reduce liver inflammation and treat liver fibrosis. In addition to hepatic differentiation, ASCs under in vitro conditions can differentiate in insulin, glucagon, and somatostatin producing cells93,94.
Facial sculpting
Apoptosis and cellular senescence (damaged cells that have lost the ability to divide) are considered important factors in ageing. Dead cells are replaced by new in the process of regeneration. They originate from stem cells, which proliferate and differentiate to maintain constant regeneration of damaged and apoptotic cells and tissues. Unfortunately, as we age, their number and proliferation capacity diminish because of senescence leading to irreversible facial cell and tissue damage and visible signs of ageing. Traditionally, dermatologists and other aesthetic healthcare professionals use and combine various methods to improve visible signs of facial skin ageing that have to be repeated at regular intervals, can be expensive, and require the commitment of patients to achieve a satisfactory outcome. Currently, signs of facial skin ageing can be temporarily improved, but, as yet, ageing itself cannot be decelerated or reversed.
Apoptosis and cellular senescence (damaged cells that have lost the ability to divide) are considered important factors in ageing.
New concepts to achieve rejuvenation of the skin, deceleration or even reverse skin ageing could be achieved by elimination of senescent cells and their epigenetic reprogramming, which is not yet fully understood. Alternatively, resetting the ‘ageing-clock’ and improvement of chronological ageing can be achieved by replacing stem cells through local administration in the face, which is currently a treatment used in aesthetic medicine today95.
Autologous adipose-derived stem cells are most commonly used to address processes of facial ageing because they can differentiate into all three embryonic tissues, their number (per gram of processed adipose tissue) is significantly higher than in bone marrow, and they are easily obtained. Routinely, 1 x 107 adipose stromal/stem cells have been isolated from 300 ml of lipoaspirate, with greater than 95% purity96. The average amount of ASCs in processed lipoaspirate is 2% of all nucleated cells97.
Autologous adipose-derived stem cells can be harvested by minimal liposuction performed under tumescent anaesthesia. The most common anatomical regions for liposuction are the abdomen, the inner thighs and the flank. Harvested lipoaspirate is subsequently processed before being administered in the face. Lipoaspirate is prepared according to desirable treatment goals: volume replacement requires administration of viable cells (adipocytes and SVF/ASC), while the regeneration of the skin requires administration of fluid consistent of growth factors and cytokines released from broken adipocytes and other SVF cellular constituents. Viable adipocytes, pericytes, and ASC are key for successful volume restoration; therefore, their manipulation must be handled with care to maximise viability and engraftment. In addition, it is very important to choose the correct harvesting and injecting cannulas in order to provide a sufficient number of viable adipocytes and SVF/ASC cells. This will prevent big cellular clusters and their core necrosis or prevent the breakdown of cells and consequently no volume restoration.
They can be easily harvested by minimal liposuction in tumescent anaesthesia, processed and administered locally in the face without previous genetic manipulation or expansion in in vitro conditions.
The transplantation of viable adipocytes and SVF/ASC-enriched fat grafts, therefore, provides a combination of volume restoration and skin regeneration98–100. Successful engraftment of adipocytes and effective regeneration of the skin can be achieved by injecting small clusters of adipose tissue (0.2 mm–0.8 mm), superficially (in the subcutaneous plane), and via a linear fanning technique (multiple tunneling) to maximise the contact surface area and provide sufficient vascular supply and ensure the survival of fat grafts101,102. Larger globules of transplanted fat undergo central necrosis, volume loss, and may result in oil cysts103,104. There is no risk of skin irregularities when fat grafts are injected superficially (in the subcutaneous plane) with a needle (21–27G) or a thin microcannula (0.8–0.4 mm).
Conclusion
Adult adipose-derived stem cells are an ideal tool in anti-ageing and regenerative medicine because they can differentiate to all three embryonic tissues and secrete cytokines and growth factors, which act in a paracrine fashion. Their number per gram of processed adipose tissue is much higher than in bone marrow, which is another advantage. They can be easily harvested by minimal liposuction in tumescent anaesthesia, processed and administered locally in the face without previous genetic manipulation or expansion in in vitro conditions. The entire procedure can be completed in a few hours and patients can leave the clinic the same day. There are no serious downsides except facial swelling for a few days and patients can return to their normal activities straight away. From an aesthetic point of view, they provide satisfactory long-term and sustainable cosmetic outcomes and may potentially replace combined cosmetic procedures, which need to be repeated regularly to achieve a comparable outcome.
Declaration of interest: The author would like to acknowledge Swiss Stem Cell Biotech as a source of information presented in the manuscript
Figure 1 © Dr Dimitra Dasiou
References
- Butler RN, Miller RA, Perry D et al. New model of health promotion and disease prevention for the 21st century. BMJ 2008;337:a339
- Ernster VL, Grady D, Miike R et al. Facial wrinkling in men and women, by smoking status. Am J Public Health 1995;85:78-82
- Forage MA, Miller KW, Elsner P et al. Intrinsic and extrinsic factors in skin ageing: a review. Int J Cosmet Sci 2008;30:87-95
- Sinclair DA, Oberdoerffer P. The ageing epigenome: Damaged beyond repair? Ageing Res Rev 2009;8:189-98
- Beckman KB, Ames BN. The free radical theory of ageing matures. Physio Rev 1998;78:547-81
- Farage MA, Miller KW, Maibach HI. Textbook of ageing skin. Berlin: Springer-Verlag, 2010
- Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin ageing. Clin Dermatol 2011;29:3-14
- Belicchi M, Pisati F, Lopa R et al. Human skin-derived stem cells migrate throughout forebrain and differentiate into astrocytes after injection into adult mouse brain. J Neurosci Res 2004;77:475–86
- Feng J, Mantesso A, Sharpe PT. Perivascular cells as mesenchymal stem cells. Expert Opin Biol Ther 2010;10:1441–51
- Lenoir N. Europe confronts the embryonic stem cell research challenge. Science 2000;287:1425–7
- Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer 2011;11:268–77.
- Song L, Young NJ, Webb NE et al. Origin and characterization of multipotential mesenchymal stem cells derived from adult human trabecular bone. Stem Cells Dev 2005;14:712–21
- Choi YS, Noh SE, Lim SM et al. Multipotency and growth characteristic of periosteum-derived progenitor cells for chondrogenic, osteogenic, and adipogenic differentiation. Biotechnol Lett 2008;30:593–601.
- De Bari C, Dell’Accio F, Tylzanowski P et al. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum 2001;44:1928–42
- Dodson MV, Hausman GJ, Guan L et al. Skeletal muscle stem cells from animals I. Basic cell biology. Int J Biol Sci2010;6:465–74
- Belicchi M, Pisati F, Lopa R et al. Human skin-derived stem cells migrate throughout forebrain and differentiate into astrocytes after injection into adult mouse brain. J Neurosci Res 2004;77:475–86
- Feng J, Mantesso A, Sharpe PT. Perivascular cells as mesenchymal stem cells. Expert Opin Biol Ther 2010;10:1441–51
- Shi M, Ishikawa M, Kamei N et al. Acceleration of skeletal muscle regeneration in a rat skeletal muscle injury model by local injection of human peripheral blood-derived cd133-positive cells. Stem Cells 2009;27:949–60
- Miura M, Gronthos S, Zhao M et al. Shed: Stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA 2003;100:5807–12
- Seo BM, Miura M, Gronthos S et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004;364:149–55
- Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007;25:1384–92
- Musina RA, Bekchanova ES, Sukhikh GT. Comparison of mesenchymal stem cells obtained from different human tissues. Bull Exp Biol Med 2005;139:504–9
- Zuk PA, Zhu M, Mizuno H et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng 2001;7:211–28
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res 2007;100:1249–60
- Tobita M, Orbay H, Mizuno H. Adipose-derived stem cells: Current findings and future perspectives. Discov Med 2011;11:160–70
- Yoshimura K, Suga H, Eto H. Adipose-derived stem/progenitor cells: Roles in adipose tissue remodeling and potential use for soft tissue augmentation. Regen Med 2009;4:265–73
- Weisberg SP, McCann D, Desai M et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–808
- Xu H, Barnes GT, Yang Q et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112: 1821–30
- Schipper BM, Marra KG, Zhang W et al. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg 2008;60:538–44
- Prunet-Marcassus B, Cousin B, Caton D et al. From heterogeneity to plasticity in adipose tissues: Site-specific differences. Exp Cell Res 2006;312:727–36
- Seale P, Bjork B, Yang W et al. Prdm16 controls a brown fat/skeletal muscle switch. Nature 2008;454:961–7
- Zimmerlin L, Donnenberg VS, Pfeifer ME et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry A 2010;77:22–30
- Bailey AM, Kapur S, Katz AJ. Characterization of adipose-derived stem cells: An update. Curr Stem Cell Res Ther 2010;5:95–102
- Basciano L, Nemos C, Foliguet B et al. Long term culture of mesenchymal stem cells in hypoxia promotes a genetic program maintaining their undifferentiated and multipotent status. BMC Cell Biol 2011;12:12
- Bernardo ME, Zaffaroni N, Novara F et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res 2007;67: 9142–9
- Deans RJ, Moseley AB. Mesenchymal stem cells: Biology and potential clinical uses. Exp Hematol 2000;28:875–84
- Folgiero V, Migliano E, Tedesco M et al. Purification and characterization of adipose-derived stem cells from patients with lipoaspirate transplant. Cell Transplant 2010;19:1225–35
- Freitas CS, Dalmau SR. Multiple sources of non-embryonic multipotent stem cells: Processed lipoaspirates and dermis as promising alternatives to bone-marrow-derived cell therapies. Cell Tissue Res 2006;325:403–11
- Gronthos S, Franklin DM, Leddy HA et al. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol 2001;189:54–63
- Jones EA, English A, Kinsey SE et al. Optimization of a flow cytometry-based protocol for detection and phenotypic characterization of multipotent mesenchymal stromal cells from human bone marrow. Cytometry B Clin Cytom 2006;70:391–9
- Katz AJ, Tholpady A, Tholpady SS et al. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hadas) cells. Stem Cells 2005;23:412–23
- Meyerrose TE, De Ugarte DA, Hofling AA et al. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells 2007;25:220–7
- Mitchell JB, McIntosh K, Zvonic S et al. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 2006;24:376–85
- Yoshimura K, Shigeura T, Matsumoto D et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol 2006;208:64–76
- Alipour R, Sadeghi F, Hashemi-Beni B et al. Phenotypic characterizations and comparison of adult dental stem cells with adiposederived stem cells. Int J Prev Med 2010;1:164–71
- Delorme B, Ringe J, Gallay N et al. Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood 2008;111:2631–35
- Kim YJ, Yu JM, Joo HJ et al. Role of cd9 in proliferation and proangiogenic action of human adipose-derived mesenchymal stem cells. Pflugers Arch 2007;455: 283–96
- Wagner W, Wein F, Seckinger A et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 2005;33:1402–16
- Suga H, Matsumoto D, Eto H et al. Functional implications of cd34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev 2009;18:1201–10
- Tang W, Zeve D, Suh JM et al. White fat progenitor cells reside in the adipose vasculature. Science 2008;322:583–6
- Traktuev DO, Merfeld-Clauss S, Li J et al. A population of multipotent cd34 positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res 2008;102: 77–85
- Jeon BG, Kumar BM, Kang EJ et al. Characterization and comparison of telomere length, telomerase and reverse transcriptase activity and gene expression in human mesenchymal stem cells and cancer cells of various origins. Cell Tissue Res 2011;345:149–61
- Puissant B, Barreau C, Bourin P et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br J Haematol 2005;129:118–29
- Van Poll D, Parekkadan B, Borel Rinkes I et al. Mesenchymal stem cell therapy for protection and repair of injured vital organs. Cell Mol Bioeng 2008;1:42–5055.
- Kim WS, Park BS, Sung JH. Protective role of adipose-derived stem cells and their soluble factors in photoageing. Arch Dermatol Res 2009;301:329–3656.
- Rehman J, Traktuev D, Li J et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004;109:1292–857.
- Salgado AJ, Reis RL, Sousa NJ et al. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther 2010;5:103–1058.
- Kilroy GE, Foster SJ, Wu X et al. Cytokine profile of human adipose-derived stem cells: Expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol 2007;212:702–9
- De Ugarte DA, Morizono K, Elbarbary A et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 2003;174:101–9
- Izadpanah R, Trygg C, Patel B et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem 2006;99:1285–97
- Rodriguez AM, Elabd C, Delteil F et al. Adipocyte differentiation of multipotent cells established from human adipose tissue. Biochem Biophys Res Commun 2004;315:255–63
- Hebert TL, Wu X, Yu G et al. Culture effects of epidermal growth factor (egf) and basic fibroblast growth factor (bfgf) on cryopreserved human adipose-derived stromal/stem cell proliferation and adipogenesis. J Tissue Eng Regen Med 2009;3: 553–61
- Kras KM, Hausman DB, Martin RJ. Tumor necrosis factor-alpha stimulates cell proliferation in adipose tissue-derived stromal-vascular cell culture: Promotion of adipose tissue expansion by paracrine growth factors. Obes Res 2000;8:186–93
- Zaragosi LE, Ailhaud G, Dani C. Autocrine fibroblast growth factor 2 signaling is critical for self-renewal of human multipotent adiposederived stem cells. Stem Cells 2006;24:2412–9
- Kang YJ, Jeon ES, Song HY et al. Role of c-jun N-terminal kinase in the pdgf-induced proliferation and migration of human adipose tissue-derived mesenchymal stem cells. J Cell Biochem 2005; 95: 1135–45.
- Song HY, Jeon ES, Jung JS et al. Oncostatin m induces proliferation of human adipose tissue-derived mesenchymal stem cells. Int J Biochem Cell Biol 2005;37:2357–65
- Kakudo N, Minakata T, Mitsui T et al. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg 2008;122:1352–60
- Blande IS, Bassaneze V, Lavini-Ramos C et al. Adipose tissue mesenchymal stem cell expansion in animal serum-free medium supplemented with autologous human platelet lysate. Transfusion 2009;49:2680–5
- Freyberg S, Song YH, Muehlberg F et al. Thrombin peptide (tp508) promotes adipose tissue-derived stem cell proliferation via pi3 kinase/akt pathway. J Vasc Res 2009;46:98–102
- Mizuno H. Adipose-derived stem and stromal cells for cell-based therapy: Current status of preclinical studies and clinical trials. Curr Opin Mol Ther 2010;12:442–9
- Zuk PA. The adipose-derived stem cell: Looking back and looking ahead. Mol Biol Cell 2010;21:1783–7
- Brayfield CA, Marra KG, Rubin JP. Adipose tissue regeneration. Curr Stem Cell Res Ther 2010;5:116–21
- Cherubino M, Marra KG. Adipose-derived stem cells for soft tissue reconstruction. Regen Med 2009;4:109–17
- Rubin JP, Marra KG. Soft tissue reconstruction. Methods Mol Biol 2011;702: 395–400
- Dragoo JL, Samimi B, Zhu M et al. Tissue-engineered cartilage and bone using stem cells from human infrapatellar fat pads. J Bone Joint Surg Br 2003;85:740–7
- Estes BT, Diekman BO, Gimble JM et al. Isolation of adipose derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc 2010;5:1294–311
- Estes BT, Guilak F. Three-dimensional culture systems to induce chondrogenesis of adipose-derived stem cells. Methods Mol Biol 2011;702:201–17
- Mizuno H, Zuk PA, Zhu M et al. Myogenic differentiation by human processed lipoaspirate cells. Plast Reconstr Surg 2002;109:199–209;discussion 210–191
- Lee WC, Sepulveda JL, Rubin JP et al. Cardiomyogenic differentiation potential of human adipose precursor cells. Int J Cardiol 2009;133:399–401
- Planat-Benard V, Menard C, Andre M et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res 2004;94:223–9
- Cherubino M, Rubin JP, Miljkovic N et al. Adipose-derived stem cells for wound healing applications. Ann Plast Surg 2011;66:210–15
- Uysal AC, Mizuno H. Tendon regeneration and repair with adipose derived stem cells. Curr Stem Cell Res Ther 2010; 5: 161–7.
- Tobita M, Mizuno H. Periodontal disease and periodontal tissue regeneration. Curr Stem Cell Res Ther 2010;5:168–74
- Brzoska M, Geiger H, Gauer S et al. Epithelial differentiation of human adipose tissue-derived adult stem cells. Biochem Biophys Res Commun 2005;330:142–50
- Long JL, Zuk P, Berke GS et al. Epithelial differentiation of adipose-derived stem cells for laryngeal tissue engineering. Laryngoscope 2010;120:125–31
- Vossmerbaeumer U, Ohnesorge S, Kuehl S et al. Retinal pigment epithelial phenotype induced in human adipose tissue-derived mesenchymal stromal cells. Cytotherapy 2009;11:177–88
- Safford KM, Safford SD, Gimble JM et al. Characterization of neuronal/glial differentiation of murine adipose-derived adult stromal cells. Exp Neurol 2004;187: 319–28
- Ikegame Y, Yamashita K, Hayashi S et al. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 2011;13:675–85
- Kim JM, Lee ST, Chu K et al. Systemic transplantation of human adipose stem cells attenuated cerebral inflammation and degeneration in a hemorrhagic stroke model. Brain Res 2007;1183:43–50
- Kang SK, Shin MJ, Jung JS et al. Autologous adipose tissue-derived stromal cells for treatment of spinal cord injury. Stem Cells Dev 2006;15:583–94
- Aurich H, Sgodda M, Kaltwasser P et al. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut 2009;58:570–81
- Banas A, Teratani T, Yamamoto Y et al. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol 2009;24:70–7
- Chandra V, Swetha G, Phadnis S et al. Generation of pancreatic hormone-expressing islet-like cell aggregates from murine adipose tissue-derived stem cells. Stem Cells 2009;27:1941–53
- Timper K, Seboek D, Eberhardt M et al. Human adipose tissue derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun 2006;341:1135–40
- Sikora E, Bielak-Zmijewska A, Mosieniak G. Cellular Senescence in Ageing, Age-Related Disease and Longevity. Curr Vasc Pharmacol 2014;12: 698-706
- Boquest AC, Shahdadfar A, Brinchmann JE et al. Isolation of stromal stem cells from human adipose tissue. Methods Mol Biol 2006;325:35–46
- Strem BM, Hicok KC, Zhu M et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med 2005;54:132–41
- Rigotti G, Marchi A, Galie M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg 2007; 119(5):1409-22;discussion 23-24
- Mojallal A, Lequeux C, Shipkov C, et al. Improvement of skin quality after fat grafting: clinical observation and an animal study. Plast Reconstr Surg 2009;124(3): 765-74
- Sterodimas A, de Faria J, Nicaretta B, et al. Tissue engineering with adipose-derived stem cells (ADSCs): current and future applications. J Plast Reconstr Aesthet Surg 2010; 63(11):1886-92
- Nguyen PS, Desouches C, Gay AM, Hautier A, et al. Development of micro-injection as an innovative autologous fat graft technique: the use of adipose tissue as dermal filler. J Plast Reconstr Aesthet Surg 2012; 65(12):1692-9
- Zeltzer AA, Tonnard PL, Verpaele AM. Sharp-needle intradermal fat grafting (SNIF). Aesthet Surg J 2012; 32(5):554-61
- James IB, Coleman SR, Rubin JP. Fat, stem cells, and platelet-rich plasma. Clin Plast Surg 2016; 43(3):473-88
- Bourne DA, James IB, Wang SS, et al. The Architecture of Fat Grafting: What Lies beneath the Surface. Plast Reconstr Surg 2016; 137(3):1072-9
- Tremolada C, Colombo V, Ventura C. Adipose tissue and mesenchymal stem cells: state of the art and Lipogems® technology development. Curr Stem Cell Rep 2016; 2:304-12
- Bianchi F, Maioli M, Leonardi E, et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant 2013;22(11):2063-77







