Dr Adele Sparavigna, Dermatologist, discusses the skin benefits of the new food supplement from Professional Dietetics
Skin, the main barrier to the external environment, is subject to deterioration caused by dermatological disorders, environmental conditions and the intrinsic ageing process. This damage to both structure and function may be accelerated by smoking, alcohol consumption, and chronic sun exposure (extrinsic components). All these factors may lead to the formation of wrinkles, the appearance of brown spots and skin thickening. One effective strategy to manage the skin ageing process is adopting a healthy nutritional approach to life, maintaining a balanced diet and a good supply of food supplements. This can restore the homeostasis of macro and micronutrients and support the physiology of cells and tissues in the skin.
“Skin viscoelasticity was significantly improved on the volar surface from 1 month post treatment.”
A major claim of most cosmetic treatments is their efficiency in skin nutrition. Amino acids are unique molecules for skin nutrition because they are the only substrate really necessary for promoting the synthesis of any protein as these are the main constituents of the extracellular matrix.

The adequacy of type and number of amino acids required for protein synthesis can be predicted on the basis of the quality and quantity of amino acids present in each protein. But collagen and elastin synthesis is different because some of its amino acids should be provided in the precursors form to activate the synthetic drive by fibroblasts. Collagen and elastin synthesis is therefore efficiently maintained only when those specific amino acids are continuously available and are present in a specific ratio.
When administered orally, amino acids reach the small intestine where they are absorbed into the blood stream. Through the network of blood vessels, these free amino acids are then distributed in the human body, in particular to the dermis, where it has been proven they can remain up to 14 days. In the dermis, free amino acids provide building blocks for the formation of collagen and elastin fibres.
Nutrakos drinkable, a food supplement with 6 amino acids in a specific ratio that has been methodologically and clinically proven to guarantee an adequate and balanced response to gene expression and production, by fibroblasts, of collagen and elastin, was chosen for a pilot study.
Study objective
The primary endpoint of this study was to evaluate the moisturising and elasticising activity of ‘Nutrakos®’ Amino Acid Food Supplement, on both photo-exposed and not photo-exposed skin areas (forearm volar and dorsal surface) by non-invasive instrumental measurements.
The study was conducted on 12 female volunteers, aged 39–62 years (mean = 50), with skin photoaging and skin phototype II and III according to Fitzpatrick’s classification, whose informed consent had been obtained and the study product were taken for 1 month.
It was also the aim of this study to evaluate tolerance both by investigator and volunteers and efficacy by the volunteers using a self-assessment questionnaire.
The clinical trial obtained the independent Ethics Committee approval in 2018.
Study design
Open, pilot clinical trial, that foresaw the food supplement assumption for 1 month continuously, of 2 stickpacks/die, the first before breakfast and the second before dinner for 1 month. The study product was taken as it was or diluted in either water or a cold drink of choice. Three visits were performed during the trial: a basal visit, an intermediate visit (after 2 week-treatment) and a final visit (after 1 month-treatment).
Inclusion criteria provided healthy adult volunteers accepting to not change their habits regarding food, physical activity, body cosmetic and cleansing products and accepting not to expose their body to strong UV irradiation (UV sessions or sun bathe) during the entire duration of the study. Smokers, alcohol or drug abusers and pregnant women were excluded from the study.
Efficacy evaluation
The efficacy of the study product was established through the instrumental evaluations of skin hydration (superficial and deep hydration) and plastoelasticity (superficial and deep plastoelasticity); all evaluations were carried out at baseline (T0), after 2 weeks (T2W) and 1 (T1M) month of treatment, mono-laterally on the right or left forearm (volar and dorsal surface) according to a subjects’ randomisation list defined by the investigator before the subjects’ inclusion.
Results
The details of all obtained results are reported in the annexed graphs and tables.
No drop out and no other important event which may have interfered to the test results occurred during the study period. The statistical analysis was performed on 12 included cases, who completed the study as per protocol.
The activity of the test product at each study time was expressed in absolute values versus baseline (T0). The data processing was performed by non-parametric test (Friedman test), when the normality hypothesis was rejected by the Shapiro-Wilk normality test (threshold at 5%) or parametric test (Anova test for repeated measures), when the normality hypothesis was confirmed, both followed in case of statistically significant result by Holm-Sidak Adjusted tests.
Efficacy evaluation
Skin hydration
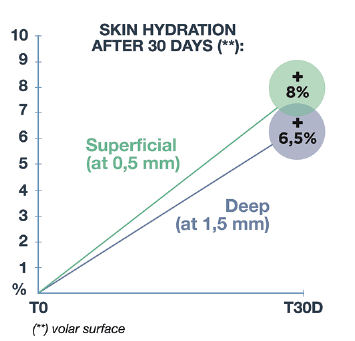
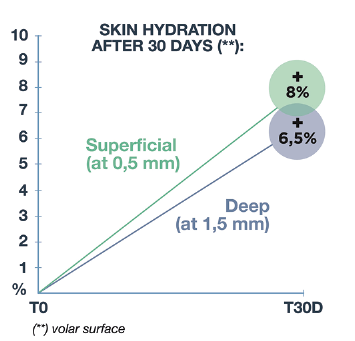
Measurements of skin hydration were performed on deep skin layers by MoistureMeterD.
Obtained results highlighted the volar surface beginning from T2W, which continues until the end of the trial T1M, a clinically/statistically significant increase of skin hydration measured at 0.5 and 1.5 mm of depth (Figure 1). Also for the dorsal surface of the forearm, where the skin photoaging is more marked, there was a trend improvement of deep hydration both at 0.5 mm (starting from T2W) and at 1.5 mm (at T1M) of depth, sign of an interesting moisturising activity, but yet not significant, in the middle-deep dermis.
Torsiometry (Skin plastoelasticity)
Superficial and deep skin plastoelasticity measurements were performed at the level of the forearm (volar and dorsal surface).
Skin viscoelasticity was significantly improved on the volar surface from 1 month post-treatment (in superficial skin and deep skin). A statistically significant improvement was noticed after 30 days in skin plastoelasticity on dorsal surface, the skin area more prone to damages of photoaging and elastosis (Figure 2). These parameters are associated to an improvement of skin tonicity/density.
Other torsiometic data did not reach statistical significance (probably because of the small sample size) but an interesting trend towards the improvement of skin plastoelasticity can be noticed.
Efficacy evaluation by the volunteers
At the end of the trial (T1M) each volunteer filled in a questionnaire regarding treatment efficacy and tolerance. Considering the sum of medium, marked and very marked judgements, it’s evident the very positive judgement of the subjects on the product firming, hydrating and smoothness efficacy.
Tolerance
No adverse event/reaction occurred during the trial, the investigator judged the tolerability of the tested product good/excellent in 100% of subjects.
Conclusions
Considering the experimental design adopted, this pilot study confirmed the moisturising and elasticising activity of ‘Nutrakos®’ Amino Acid Food Supplement on the dermis, demonstrating to recover the alteration of biological functions and ability to manage metabolic stress induced by UV radiation, smoke and pollution is one of the major consequences of the skin ageing process.
One particularly interesting finding coming from the study is that statistically significant results have been found already after only 2 weeks of intervention in terms of dermal hydration and plastoelasticity. It is important to consider that some other nutritional products, based on hydrolysed collagen, proposed for skin anti-ageing have managed to demonstrate their effectiveness after at least 4 weeks of treatment.
This superiority could result from actually identifying the standardized cluster of amino acids most suitable for inducing a protein synthesis oriented to the extra cellular matrix key proteins.
It would be interesting to confirm these preliminary results through a clinical trial on a larger population sample.
The final product tolerance was judged good/excellent, in fact no adverse reaction related to the product assumption occurred during the trial.






